CRASH COURSE MHT-CET CHEMISTRY
| Site: | eduvertex |
| Course: | CHEMISTRY FOR MHT-CET |
| Book: | CRASH COURSE MHT-CET CHEMISTRY |
| Printed by: | Guest user |
| Date: | Saturday, 18 May 2024, 12:36 PM |
Table of contents
- 1. CONCEPT MOLE CONCEPT
- 2. PRACTICE SET MOLE CONCEPT
- 3. CONCEPT: Atomic Structure
- 4. PRACTICE SET: Atomic Structure
- 5. CONCEPT: CHEMICAL BONDING
- 6. THEORY-CHEMICAL BONDING
- 7. EXERCISE-1 CHEMICAL BONDING
- 8. Theory: Redox Reaction
- 9. EXERCISE-1 Redox Reaction
- 10. Group I & II
- 11. Exercise Group I & II
- 12. Theory States of matter
- 13. Exercise: States of matter
- 14. Solid States
- 15. Solutions and colligative properties
- 16. Chemical thermodynamics
- 17. Electrochemistry
- 18. Chemical Kinetics
- 19. General principle and process of isolation of elements
- 20. P BLOCK ELEMENTS
- 21. D AND F BLOCK ELEMENTS
- 22. CO-ORDINATION COMPOUNDS
- 23. Halogens derivatives of alkanes and arenes
- 24. Alcohols, phenols and ethers
- 25. Aldehydes ketones and carboxylic acids
- 26. compounds containing nitrogen
- 27. Biomolecules
- 28. Polymers
- 29. Chemistry in everyday life
1. CONCEPT MOLE CONCEPT
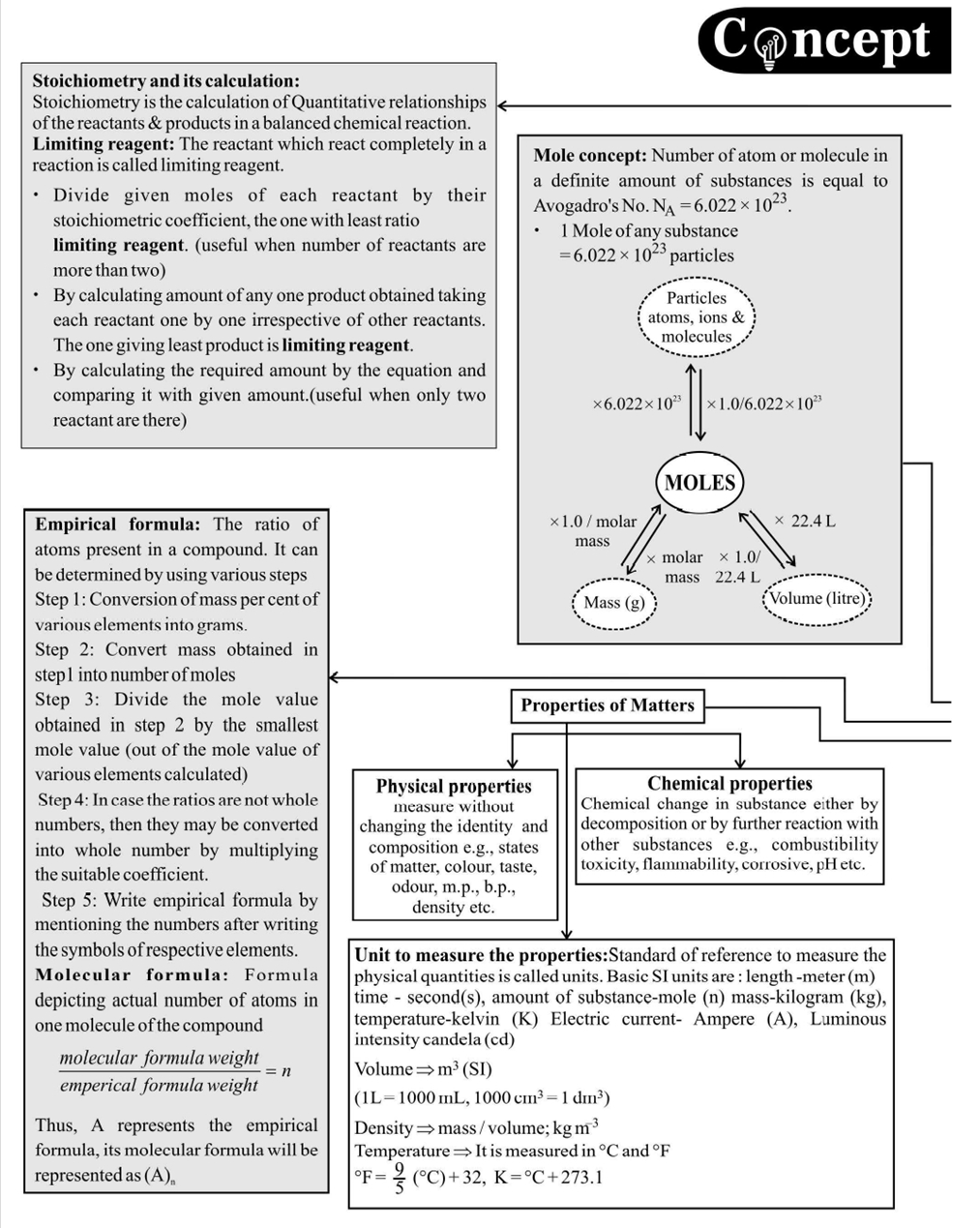
Important Points to Remember
- Dulong and Pettit's method
Atomic mass \(\times\) Specific heat \(=6.4\) (approx.)
This law is applicable to solid elements only except Be, B, C and \(\mathrm{Si}\)
- On diluting a solution, eq, meq, mole or \(\mathrm{m}\) mole of solute do not change however \(\mathrm{N}\) and \(\mathrm{M}\) change (decrease).
- Molality, \% by weight, mole fraction do not depend on temperature since they involve weights.
- Normality, molarity, percent of volume; percent by strength, and strength are temperature dependent. They decrease with increase in temperature since the volume of solution increases with \(\mathrm{T}\).
- Sometimes, the term formality is used in place of molarity.
- Standard solution is one whose \(\mathrm{N}\) or \(\mathrm{M}\) is known.
- Normality: It is defined as number of equivalents of a solute present in one litre of solution. \(\mathrm{N}\) = Equivalent of solute/ Volume of solution (L)
\(\mathrm{N}=\frac{\text { Weight of solution }}{\text { Equivalent weight of solute } \times \mathrm{V}_{\text {sol }}(\mathrm{inL})}\)
\(\mathrm{N}=\frac{\mathrm{W}}{\mathrm{E} \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{L})}=\frac{\mathrm{W} \times 1000}{\mathrm{E} \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{mL})}\)
- Molarity: It is defined as the moles of solute present in one litre of solution.
\(\mathrm{M}=\frac{\text { Moles of solute }}{\text { Volume of solution in litre }}\)
\(M=\frac{\text { Weight of solute }}{\text { Molecular weight of solute } \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{L})}\)
- Molality: Moles of solute present in \(1 \mathrm{~kg}\) of solvent.
Molality \(=\) Moles of solute/ Weight of solvent in \(\mathrm{kg}\)
D Vapour density method
Tips to Problem Solving
\(n\)-Factor
Molecular weight \(=2 \times\) vapour density
Equivalent weight \(=\) atomic weight \(/ n\)-factor
In case of acid/base the \(n\)-factor is basicity/acidity (i.e. number of dissociable \(\mathrm{H}^{+}\)ions/number of dissociable \(\mathrm{OH}^{-}\)ions) and in case of oxidizing agent/reducing agent, \(n\) factor is number of moles of electrons gained/lost per mole of oxidizing agent/reducing agent.
" Molality \((m)\) expressed in terms of mole fraction of solute \(\left(X_{1}\right)\) is given by relation: \(m=\frac{1000 X_{1}}{\left(1-X_{1}\right) m_{2}}\) where \(\mathrm{m}_{2}\) is the molar mass of the solvent.
" Molality \((m)\) is related to molarity \((M)\) by relation : \(\mathrm{m}=\frac{1000 \mathrm{M}}{1000 \mathrm{~d}-\mathrm{Mm}_{1}}\) where \(d\) is the density \(\left(\mathrm{g} \mathrm{mL}^{-1}\right)\) and \(m 1\) is the molar mass of the solute
" Percentage:
\%by weight \(=\frac{\text { Weight of solute }}{\text { Weight of solute }} \times 100\)
\%by volume \(=\frac{\text { volume of solute }}{\text { Volume of solution }} \times 100\)
\(\%\) by \((\) W \(/ \mathrm{V})=\frac{\text { Weight of solute }}{\text { Volume of solution }} \times 100\)
2. PRACTICE SET MOLE CONCEPT
\section{TOPIC 1: Measurement, Significant Figures and Laws of Chemical Combination}
1. Which has highest weight?
1) \(1 \mathrm{~m}^{3}\) of water
2) A normal adult man
3) 10 litre of \(\mathrm{Hg}\)
4) All have same weight
2. Arrange the numbers in increasing no. of significant figures. \(0.002600,2.6000,2.6,0.260\)
1) \(2.6<0.260<0.002600<2.6000\)
2) \(2.6000<2.6<0.002600<0.260\)
3) \(0.260<2.6<0.002600<2.6000\)
4) \(0.002600<0.260<2.6<2.6000\)
3. In which of the following number all zeros are significant?
1) 0.0005
2) 0.0500
3) 50.000
4) 0.0050
4. Which of the following is the best example of law of conservation of mass?
1) \(12 \mathrm{~g}\) of carbon combines with \(32 \mathrm{~g}\) of oxygen to form \(44 \mathrm{~g}\) of \(\mathrm{CO}_{2}\).
2) When \(12 \mathrm{~g}\) of carbon is heated in a vacuum there is no change in mass.
3) A sample of air increases in volume when heated at constant pressure but its mass remains unaltered.
4) The weight of a piece of platinum is the same before and after heating in air.
5. The percentage of copper and oxygen in samples of \(\mathrm{CuO}\) obtained by different methods were found to be the same. This illustrates the law of
1) constant proportions 2 ) conservation of mass
3) multiple proportions
4) reciprocal proportions
6. One of the following combination which illustrates the law of reciprocal proportions?
1) \(\mathrm{N}_{2} \mathrm{O}_{3}, \mathrm{~N}_{2} \mathrm{O}_{4}, \mathrm{~N}_{2} \mathrm{O}_{5}\) 2) \(\mathrm{NaCl}, \mathrm{NaBr}, \mathrm{NaI}\)
3) \(\mathrm{CS}_{2}, \mathrm{CO}_{2}, \mathrm{SO}_{2}\)
4) \(\mathrm{PH}_{3}, \mathrm{P}_{2} \mathrm{O}_{3}, \mathrm{P}_{2} \mathrm{O}_{5}\)
7. The molecular weight of \(\mathrm{O}_{2}\) and \(\mathrm{SO}_{2}\) are 32 and 64 respectively. At \(15^{\circ} \mathrm{C}\) and \(150 \mathrm{~mm} \mathrm{Hg}\) pressure, one litre of \(\mathrm{O}_{2}\) contains ' \(\mathrm{N}\) ' molecules. The number of molecules in two litres of \(\mathrm{SO}_{2}\) under the same conditions of temperature and pressure will be :
1) \(N / 2\)
2) \(1 \mathrm{~N}\)
3) \(2 \mathrm{~N}\)
4) \(4 \mathrm{~N}\)
8. \(10 \mathrm{dm}^{3}\) of \(\mathrm{N}_{2}\) gas and \(10 \mathrm{dm}^{3}\) of gas \(\mathrm{X}\) at the same temperature contain the same number of molecules, the gas \(\mathrm{X}\) is
1) \(\mathrm{CO}_{2}\)
2) \(\mathrm{CO}\)
3) \(\mathrm{H}_{2}\)
4) \(\mathrm{NO}\)
9. What is the mass of 1 molecule of \(\mathrm{CO}\) ?
\section{TOPIC 2: Mole Concept}
1) \(2.325 \times 10^{-23}\)
2) \(4.65 \times 10^{-23}\)
3) \(3.732 \times 10^{-23}\)
4) \(2.895 \times 10^{-23}\)
10. How many atoms are contained in one mole of sucrose \(\left(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11}\right)\) ?
1) \(20 \times 6.02 \times 10^{23}\) atoms \(/ \mathrm{mol}\)
2) \(45 \times 6.02 \times 10^{23}\) atoms \(/ \mathrm{mol}\)
3) \(5 \times 6.02 \times 10^{23}\) atoms \(/ \mathrm{mol} \mathrm{4)} \mathrm{None} \mathrm{of} \mathrm{these}\)
11. Molecular mass is defined as the
1) mass of one atom compared with the mass of one molecule
2) mass of one atom compared with the mass of one atom of hydrogen
3) mass of one molecule of any substance compared with the mass of one atom of C-12
4) None of the above
12. Two containers \(\mathrm{P}\) and \(\mathrm{Q}\) of equal volume (1 litre each) contain \(6 \mathrm{~g}\) of \(\mathrm{O}_{2}\) and \(\mathrm{SO}_{2}\) respectively at \(300 \mathrm{~K}\) and 1 atmosphere, then
1) number of molecules in \(P\) is less than that in \(Q\).
2) number of molecules in \(P\) and \(Q\) is same.
3) number of molecules in \(Q\) is less than that in \(P\).
4) either 1) or 2 ).
13. A sample of \(\mathrm{AlF}_{3}\) contains \(3.0 \times 10^{24} \mathrm{~F}^{-}\)ions. The number of formula unit of this sample are
1) \(9 \times 10^{24}\)
2) \(3 \times 10^{24}\)
3) \(0.75 \times 10^{24}\)
4) \(1.0 \times 10^{24}\)
14. Which one of the following is the lightest?
1) 0.2 mole of hydrogen gas
2) \(6.023 \times 10^{22}\) molecules of nitrogen
3) \(0.1 \mathrm{~g}\) of silver
4) 0.1 mole of oxygen gas
15. The average atomic mass of neon based on following data is:
\(\begin{array}{ll}\text { Isotope } & \text { Relative abundance } \\ { }^{20} \mathrm{Ne} & 0.9051 \\ { }^{21} \mathrm{Ne} & 0.0027 \\ { }^{22} \mathrm{Ne} & 0.0922\end{array}\)
1) \(0.33 \mathrm{u}\)
2) \(20.187 \mathrm{u}\)
3) \(6.729 \mathrm{u}\)
4) \(18.058 \mathrm{u}\)
16. The ratio of number of oxygen atoms \((\mathrm{O})\) in \(16.0 \mathrm{~g}\) ozone \(\left(\mathrm{O}_{3}\right), 28.0\) g carbon monoxide (CO) and 16.0 oxygen \(\left(\mathrm{O}_{2}\right)\) is (Atomic mass : \(\mathrm{C}=12, \mathrm{O}=16\) and Avogadro's constant \(\mathrm{NA}=6.023 \times 10^{23} \mathrm{~mol}^{-1}\) )
1) \(3: 1: 2\)
2) \(1: 1: 2\)
3) \(3: 1: 1\)
4) \(1: 1: 1\)
17. A mixture of \(\mathrm{CH}_{4}, \mathrm{~N}_{2}\) and \(\mathrm{O}_{2}\) is enclosed in a container of 1 litre capacity at \(0^{\circ} \mathrm{C}\). Total pressure of gaseous mixture is \(2660 \mathrm{~mm} \mathrm{Hg}\). If the ratio of partial pressures of the gases is \(1: 4: 2\) respectively, the number of moles of oxygen present in the vessel is:
1)1/ 22.4
2) 1.0
3) 0.1
4) none of these
18. The number of gram molecules of oxygen in \(6.02 \times 10^{24} \mathrm{CO}\) molecules is
1) 10 g molecules 2) 5 g molecules
3) \(1 \mathrm{~g}\) molecules
4) 0.5 g molelcules
19. The number of molecules in \(16 \mathrm{~g}\) of methane is
1) \(3.0 \times 10^{23}\)
2) \(\frac{16}{6.02} \times 10^{23}\)
3) \(6.023 \times 10^{23}\)
4) \(\frac{16}{3.0} \times 10^{23}\)
20. \(25.4 \mathrm{~g}\) of \(\mathrm{I}_{2}\) and \(14.2 \mathrm{~g}\) of \(\mathrm{Cl}_{2}\) are made to react completely to yield a mixture of ICl and \(\mathrm{ICl}_{3} . \mathrm{Calculate}^{2}\) moles of \(\mathrm{ICl}\) and \(\mathrm{ICl}_{3}\) formed
1) \(0.1,0.1\)
2) \(0.2,0.2\)
3) \(0.1,0.2\)
4) \(0.2,0.1\)
21. If we consider that \(1 / 6\), in place of \(1 / 12\), mass of carbon atom is taken to be the relative atomic mass unit, the mass of one mole of a substance will
1) decrease twice
2) increase two fold
3) remain unchanged
4) be a function of the molecular mass of the substance
22. Number of moles of \(\mathrm{MnO}^{4-}\) required to oxidize one mole of ferrous oxalate completely in acidic medium will be
1) 0.6 moles
2) 0.4 moles
3) 7.5 moles
4) 0.2 moles
23. The number of moles of oxygen in one litre of air containing \(21 \%\) oxygen by volume, under standard conditions are
1) 0.0093 mole
2) 0.21 mole
3) 2.10 mole
4) 0.186 mole
24. Haemoglobin contains \(0.334 \%\) of iron by weight. The molecular weight of haemoglobin is approximately 67200. The number of iron atoms (at. wt. of Fe is 56) present in one molecule of haemoglobin are
1) 1
2) 6
3) 4
4) 2
25. How many moles of \(\mathrm{P}_{4} \mathrm{O}_{6}\) and \(\mathrm{P}_{4} \mathrm{O}_{10}\) will be produced by the combustion of \(12.4 \mathrm{~g}\) of phosphorous (atomic mass 31) in \(12.8 \mathrm{~g}\) of oxygen, leaving no \(\mathrm{P}_{4}\) or \(\mathrm{O}_{2}\) ?
1) 0.1 and \(0.3 \mathrm{~mol}\)
2) \(0.15 \mathrm{~mol}\) and \(0.25 \mathrm{~mol}\)
3) \(0.05 \mathrm{~mol}\) each
4) \(0.1 \mathrm{~mol}\) each
\section{TOPIC 3: Atomic, Molecular Masses, Equivalent Masses and Expression of Concentration}
26. Number of atoms in \(558.5 \mathrm{~g} \mathrm{Fe}\) (at. wt. of \(\mathrm{Fe}=55.85 \mathrm{~g} \mathrm{~mol}^{-1}\) ) is
1) twice that in 60 g carbon
2) \(6.023 \times 10^{22}\)
3) half that in \(8 \mathrm{~g} \mathrm{He}\)
4) \(558.5 \times 6.023 \times 10^{23}\)
27. The percentage weight of \(\mathrm{Zn}\) in white vitriol \(\left[\mathrm{ZnSO}_{4} .7 \mathrm{H}_{2} \mathrm{O}\right]\) is approximately equal to \((\mathrm{Zn}=65, \mathrm{~S}=32\), \(\mathrm{O}=16\) and \(\mathrm{H}=1)\)
1) \(33.65 \%\)
2) \(32.56 \%\)
3) \(23.65 \%\)
4) \(22.65 \%\)
28. The vapour density of a mixture containing \(\mathrm{NO}_{2}\) and \(\mathrm{N}_{2} \mathrm{O}_{4}\) is 27.6 . Mole fraction of \(\mathrm{NO}_{2}\) in the mixture is
1) 0.2
2) 0.4
3) 0.6
4) 0.8
29. The number of molecules in 8.96 litre of a gas at \(0^{\circ} \mathrm{C}\) and 1 atm. pressure is approximately
1) \(6.023 \times 10^{23}\)
2) \(12.04 \times 10^{23}\)
3) \(18.06 \times 10^{23}\)
4) \(24.08 \times 10^{22}\)
30. Suppose two elements \(X\) and \(Y\) combine to form two compounds \(X Y_{2}\) and \(X_{3} Y_{2}\) when 0.1 mole of former weighs \(10 \mathrm{~g}\) while \(0.05 \mathrm{~mol}\) of the latter weights \(9 \mathrm{~g}\). The atomic masses of \(\mathrm{X}\) and \(\mathrm{Y}\) are respectively
1) \(60 \& 40\)
2) \(30 \& 40\)
3) \(40 \& 30\)
4) \(40 \& 60\)
31. \(4 \mathrm{~g}\) of a hydrated crystal of formula A. \(x \mathrm{H}_{2} \mathrm{O}\) has \(0.8 \mathrm{~g}\) of water. If the molar mass of the anhydrous crystal 1) is \(144 \mathrm{~g} \mathrm{~mol}^{-1}\), The value of \(\mathrm{x}\) is
1) 4
2) 1
3) 2
4) 3
32. \(6.02 \times 10^{20}\) molecules of urea are present in \(100 \mathrm{~mL}\) of its solution. The concentration of solution is
1) \(0.01 \mathrm{M}\)
2) \(0.001 \mathrm{M}\)
3) \(0.1 \mathrm{M}\)
4) \(0.02 \mathrm{M}\)
33. A metallic chloride contain \(47.22 \%\) metal. Calculate the equivalent weight of metal.
1) 39.68
2) 31.76
3) 36.35
4) 33.46
34. Sulphur forms the chlorides \(\mathrm{S}_{2} \mathrm{Cl}_{2}\) and \(\mathrm{SCl}_{2}\). The equivalent mass of sulphur in \(\mathrm{SCl}_{2}\) is
1) \(8 \mathrm{~g} / \mathrm{mol}\)
2) \(16 \mathrm{~g} / \mathrm{mol}\)
3) \(64.8 \mathrm{~g} / \mathrm{mol}\)
4) \(32 \mathrm{~g} / \mathrm{mol}\)
35. If \(0.20 \mathrm{~g}\) chloride of a certain metal, when dissolved in water and treated with excess of \(\mathrm{AgNO}_{3}\), yields \(0.50 \mathrm{~g}\) of \(\mathrm{AgCl}\), the equivalent mass of the metal is \((\mathrm{Ag}=108, \mathrm{Cl}=35.5)\)
1) 21.90
2) 20.04
3) 40.08
4) 43.80
36. Equivalent mass of a metal \(M\) is 2.5 times that of oxygen. The minimum molecular mass of its oxide is
1) 28
2) 42
3) 56
4) 112
37. The same amount of a metal combines with \(0.100 \mathrm{~g}\) of oxygen and with \(1.000 \mathrm{~g}\) of a halogen. Hence, the equivalent mass of halogen is
1) 9
2) 35.5
3) 80
4) 127
38. In the reaction \(\mathrm{NaOH}+\mathrm{Al}(\mathrm{OH})_{3} \rightarrow \mathrm{NaAlO}_{2}+\mathrm{H}_{2} \mathrm{O}\) The equivalent mass of \(\mathrm{Al}(\mathrm{OH})_{3}\) is
1) 78
2) 26
3) 52
4) unpredictable
39. On reduction \(1.644 \mathrm{~g}\) of hot iron oxide give \(1.15 \mathrm{~g}\) of iron. Evaluate the equivalent weight of iron.
1) 18.62
2) 19.13
3) 18.95
4) 12.95
40. If a pure compound is composed of \(X_{2} Y_{3}\) molecules and consists of \(60 \%\) of \(X\) by weight, the atomic weight of \(Y\) is
1) 2.25 times atomic weight of \(X\)
2) \(44 \%\) of atomic weight of \(X\)
3) 4.0 times the atomic weight of \(X\)
4) \(25 \%\) of the atomic weight of \(X\)
41. Oxalic acid \(\mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}\) reacts with permanganate ion according to the balanced equation below. How many mL of \(0.0154 \mathrm{KMnO}_{4}\) solution are required to react with \(25.0 \mathrm{~mL}\) of \(0.0208 \mathrm{M} \mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}\) solution? \(5 \mathrm{H}_{2} \mathrm{C}_{2} \mathrm{O}_{4}(\mathrm{aq})+2 \mathrm{MnO}_{4}^{-}(\mathrm{aq})+6 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow 2 \mathrm{Mn}^{2+}(\mathrm{aq})+10 \mathrm{CO}_{2}(\mathrm{~g})+8 \mathrm{H}_{2} \mathrm{O}(1)\)
1) \(13.5 \mathrm{~mL}\)
(2) \(18.5 \mathrm{~mL}\)
(3) \(33.8 \mathrm{~mL}\)
(4) \(84.4 \mathrm{~mL}\)
42. Percentage of Se in peroxidase anhydrase enzyme is \(0.5 \%\) by weight (at. wt. of Se \(=78.4)\) then minimum molecular weight of peroxidase anhydrase enzyme is
1) \(1.568 \times 10^{3}\)
2) 15.68
3) \(2.136 \times 10^{4}\)
4) \(1.568 \times 10^{4}\)
43. Liquid benzene \(\left(\mathrm{C}_{6} \mathrm{H}_{6}\right)\) burns in oxygen according to the equation
\[
2 \mathrm{C}_{6} \mathrm{H}_{6}(1)+15 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 12 \mathrm{CO}_{2}(\mathrm{~g})+6 \mathrm{H}_{2} \mathrm{O}(\mathrm{g})
\]
How many litres of \(\mathrm{O}_{2}\) at STP are needed to complete the combustion of 39 g of liquid benzene?(Mol. wt. of \(\left.\mathrm{O}_{2}=\quad 32, \mathrm{C}_{6} \mathrm{H}_{6}=78\right)\)
1) \(74 \mathrm{~L}\)
2) \(11.2 \mathrm{~L}\)
3) \(22.4 \mathrm{~L}\)
4) \(84 \mathrm{~L}\)
44. The mass of \(\mathrm{BaCO}_{3}\) produced when excess \(\mathrm{CO} 2\) is bubbled through a solution of \(0.205 \mathrm{~mol} \mathrm{Ba}(\mathrm{OH})_{2}\) is
1) \(81 \mathrm{~g}\)
2) \(40.5 \mathrm{~g}\)
3) \(20.25 \mathrm{~g}\)
4) \(162 g\)
45. \(12 \mathrm{~L}\) of \(\mathrm{H}_{2}\) and \(11.2 \mathrm{~L}\) of \(\mathrm{Cl}_{2}\) are mixed and exploded. Find the composition by volume of mixture.
1) \(11.2,11.2,22.4\)
2) \(0.8,0,22.4\)
3) \(0.8,0.8,22.4\)
4) \(0.8,11.2,22.4\)
46. 10 moles \(\mathrm{SO}_{2}\) and 15 moles \(\mathrm{O}_{2}\) were allowed to react over a suitable catalyst. 8 moles of \(\mathrm{SO}_{3}\) were formed. The remaining moles of \(\mathrm{SO}_{2}\) and \(\mathrm{O}_{2}\) respectively are -
1) 2 moles, 11 moles
2) 2 moles, 8 moles
3) 4 moles, 5 moles
4) 8 moles, 2 moles
47. How many moles of \(\mathrm{KI}\) are required to produce 0.4 moles of \(\mathrm{K}_{2} \mathrm{HgI}_{4}\) ?
1) 0.4
2) 0.8
3) 3.2
4) 1.6
48. Under similar conditions of pressure and temperature, \(40 \mathrm{~mL}\) of slightly moist hydrogen chloride gas is mixed with \(20 \mathrm{~mL}\) of ammonia gas, the final volume of gas at the same temperature and pressure will be
1) \(100 \mathrm{~mL}\)
2) \(20 \mathrm{~mL}\)
3) \(40 \mathrm{~mL}\)
4) \(60 \mathrm{~mL}\)
49. What is the volume of \(\mathrm{CO}_{2}\) liberated (in litres) at 1 atmosphere and \(0^{\circ} \mathrm{C}\) when \(10 \mathrm{~g}\) of \(100 \%\) pure calcium carbonate is treated with excess dilute sulphuric acid?
(Atomic mass Ca : 40, C:12, O : 16)
1) 0.224
2) 2.24
3) 22.4
4) 224
50. The volume of \(0.1 \mathrm{~N}\) dibasic acid sufficient to neutralize \(1 \mathrm{~g}\) of a base that furnishes 0.04 mole of \(\mathrm{OH}-\) in aqueous solution is :
1) \(400 \mathrm{~mL}\)
2) \(600 \mathrm{~mL}\)
3) \(200 \mathrm{~mL}\)
4) \(800 \mathrm{~mL}\)
51. The density of 3M solution of sodium chloride is \(1.252 \mathrm{~g} \mathrm{~mL}^{-1}\). The molality of the solution will be : (molar mass, \(\mathrm{NaCl}=58.5 \mathrm{~g} \mathrm{~mol}^{-1}\) )
1) \(260 \mathrm{~m}\)
2) \(2.18 \mathrm{~m}\)
3) \(2.79 \mathrm{~m}\)
4) \(3.00 \mathrm{~m}\)
52. The amount of \(\mathrm{BaSO}_{4}\) formed upon mixing \(100 \mathrm{~mL}\) of \(20.8 \% \mathrm{BaCl}_{2}\) solution with \(50 \mathrm{~mL}\) of \(9.8 \%\) \(\mathrm{H}_{2} \mathrm{SO}_{4}\) solution with \(50 \mathrm{~mL}\) of \(9.8 \% \mathrm{H}_{2} \mathrm{SO}_{4}\) solution will be: \((\mathrm{Ba}=137, \mathrm{Cl}=35.5, \mathrm{~S}=32, \mathrm{H}=1\) and \(\mathrm{O}=16)\)
1) \(23.3 \mathrm{~g}\)
2) \(11.65 \mathrm{~g}\)
3) \(30.6 \mathrm{~g}\)
4) \(33.2 \mathrm{~g}\)
53. The formula of an acid is \(\mathrm{HXO}_{2}\). The mass of 0.0242 moles of the acid is \(1.657 \mathrm{~g}\). What is the atomic mass of \(X\) ?
1) 35.5
2) 28.1
3) 128
4) 19.0
54. A portable hydrogen generator utilizes the reaction between calcium hydride and water to produce hydrogen. What mass of hydrogen can be produced by 70 g cartridge of calcium hydride ?
1) \(6.7 \mathrm{~g}\)
2) \(3.5 \mathrm{~g}\)
3) \(4.5 \mathrm{~g}\)
4) \(5.5 \mathrm{~g}\)
55. If \(1 \frac{1}{2}\) moles of oxygen combine with \(\mathrm{Al}\) to form \(\mathrm{Al}_{2} \mathrm{O}_{3}\) the weight of \(\mathrm{Al}\) used in the reaction is \((\mathrm{Al}=27)\)
1) \(27 \mathrm{~g}\)
2) \(54 \mathrm{~g}\)
3) \(49.5 \mathrm{~g}\)
4) \(31 \mathrm{~g}\)
56. \(\mathrm{O}_{2}, \mathrm{~N}_{2}\) are present in the ratio of \(1: 4\) by weight. The ratio of number of molecules is :
1) \(7: 32\)
2) \(1: 4\)
3) \(2: 1\)
4) \(4: 1\)
57. \(6.8 \mathrm{~g} \mathrm{H}_{2} \mathrm{O}_{2}\) present in \(100 \mathrm{~mL}\) of its solution. What is the molarity of solution?
1) \(1 \mathrm{M}\)
2) \(2 \mathrm{M}\)
3) \(3 \mathrm{M}\)
4) \(0.5 \mathrm{M}\)
58. How many moles of magnesium phosphate, \(\mathrm{Mg}_{3}\left(\mathrm{PO}_{4}\right)_{2}\) will contain 0.25 mole of oxygen atoms?
1) \(1.25 \times 10^{-2}\)
2) \(2.5 \times 10^{-2}\)
3) 0.02
4) \(3.125 \times 10^{-2}\)
59. How many moles of \(\mathrm{Al}_{2}\left(\mathrm{SO}_{4}\right)_{3}\) would be in \(50 \mathrm{~g}\) of the substance ?
1) 0.083 mole
2) 0.952 mole
3) 0.481 mole
4) 0.140 mole
60. \(25 \mathrm{~mL}\) of a solution of barium hydroxide on titration with a 0.1 molar solution of hydrochloric acid gave a litre value of \(35 \mathrm{~mL}\). The molarity of barium hydroxide solution was
1) 0.14
2) 0.28
3) 0.35
4) 0.07
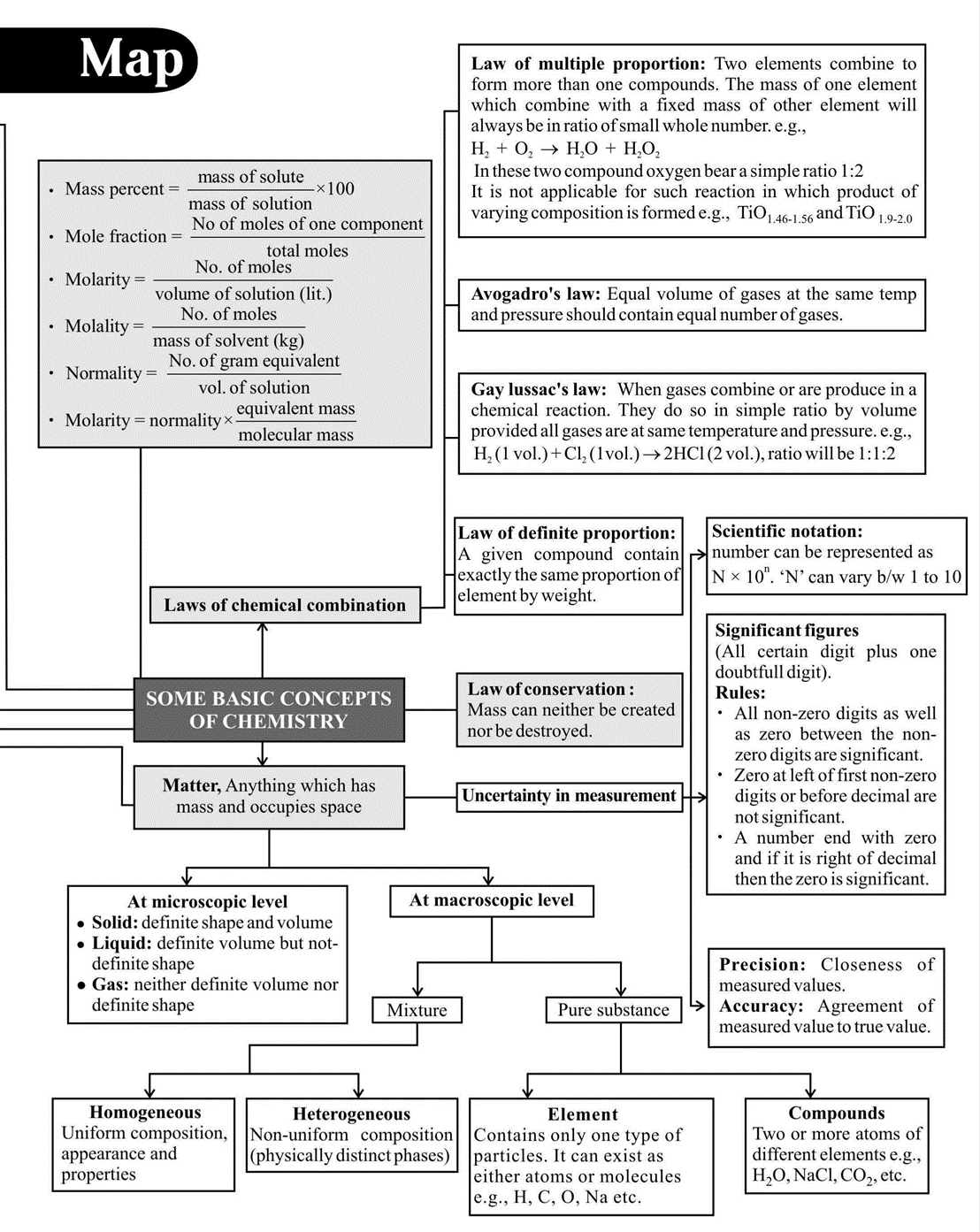
TOPIC 4: Empirical Formula, Molecular Formula and Chemical Stoichiometry
61. The empirical formula of a compound is \(\mathrm{CH}_{2}\). One mole of this compound has a mass of 42 grams. Its molecular formula is :
1) \(\mathrm{C}_{3} \mathrm{H}_{6}\)
2) \(\mathrm{C}_{3} \mathrm{H}_{8}\)
3) \(\mathrm{CH}_{2}\)
4) \(\mathrm{C}_{2} \mathrm{H}_{2}\)
62. An organic compound contains carbon, hydrogen and oxygen. Its elemental analysis gave C, 38.71\% and \(\mathrm{H}, 9.67 \%\). The empirical formula of the compound would be :
1) \(\mathrm{CH}_{3} \mathrm{O}\)
2) \(\mathrm{CH}_{2} \mathrm{O}\)
3) \(\mathrm{CHO}\)
4) \(\mathrm{CH}_{4} \mathrm{O}\)
63. In a compound \(\mathrm{C}, \mathrm{H}\) and \(\mathrm{N}\) atoms are present in \(9: 1: 3.5\) by weight. Molecular weight of compound is 108. Molecular formula of compound is
1) \(\mathrm{C}_{2} \mathrm{H}_{6} \mathrm{~N}_{2}\)
2) \(\mathrm{C}_{3} \mathrm{H}_{4} \mathrm{~N}\)
3) \(\mathrm{C}_{6} \mathrm{H}_{8} \mathrm{~N}_{2}\)
4) \(\mathrm{C}_{9} \mathrm{H}_{12} \mathrm{~N}_{3}\)
64. A chloride of a metal \((M)\) contains \(65.5 \%\) of chlorine. \(100 \mathrm{~mL}\) of vapour of the chloride of metal at STP weighs \(0.72 \mathrm{~g}\). The molecular formula of the metal chloride is :
1) \(\mathrm{MCl}\)
2) \(\mathrm{MCl}_{2}\)
3) \(\mathrm{MCl}_{3}\)
4) \(\mathrm{MCl}_{4}\)
65. In the reaction \(4 \mathrm{NH}_{3}(\mathrm{~g})+5 \mathrm{O}_{2}(\mathrm{~g}) \rightarrow 4 \mathrm{NO}(\mathrm{g})+6 \mathrm{H}_{2} \mathrm{O}(\mathrm{l})\) When 1 mole of ammonia and 1 mole of \(\mathrm{O} 2\) are made to react to completion,
1) 1.0 mole of \(\mathrm{H}_{2} \mathrm{O}\) is produced
2) 1.0 mole of NO will be produced
3) all the oxygen will be consumed
4) all the ammonia will be consumed
66. If potassium chlorate is \(80 \%\) pure, then \(48 \mathrm{~g}\) of oxygen would be produced from (atomic mass of \(\mathrm{K}=39\) )
1) \(153.12 \mathrm{~g}\) of \(\mathrm{KClO}_{3}\)
2) \(122.5 \mathrm{~g}\) of \(\mathrm{KClO}_{3}\)
3) \(245 \mathrm{~g}\) of \(\mathrm{KClO}_{3}\)
4) 98 g of \(\mathrm{KClO}_{3}\)
67. The empirical formula of an acid is \(\mathrm{CH}_{2} \mathrm{O}_{2}\), the probable molecular formula of acid may be :
1) \(\mathrm{C} 3 \mathrm{H}_{6} \mathrm{O}_{4}\)
2) \(\mathrm{CH}_{2} \mathrm{O}\)
3) \(\mathrm{CH}_{2} \mathrm{O}_{2}\)
4) \(\mathrm{C}_{2} \mathrm{H}_{4} \mathrm{O}_{2}\)
68. Which of the following is the correct empirical and molecular formulae of a compound, if the molecular mass of a compound is 80 and compound contains \(60 \%\) of \(\mathrm{C}, 5 \%\) of \(\mathrm{H}\) and \(35 \%\) of \(\mathrm{N}\) ?
1) \(\mathrm{C}_{2} \mathrm{H}_{2} \mathrm{~N} ; \mathrm{C}_{4} \mathrm{H}_{4} \mathrm{~N}_{2}\)
2) \(\mathrm{C}_{3} \mathrm{H}_{4} \mathrm{~N}_{2} ; \mathrm{C}_{6} \mathrm{H}_{8} \mathrm{~N}_{4}\)
3) \(\mathrm{C}_{2} \mathrm{H}_{4} \mathrm{~N}_{2} ; \mathrm{C}_{4} \mathrm{H}_{8} \mathrm{~N}_{4}\)
4) \(\mathrm{C}_{2} \mathrm{H}_{2} \mathrm{~N} ; \mathrm{C}_{2} \mathrm{H}_{2} \mathrm{~N}\)
69. \(12 \mathrm{gm}\) of \(\mathrm{Mg}\) (atomic mass 24) will react completely with hydrochloric acid to give
1) One mol of \(\mathrm{H}_{2}\)
2) \(1 / 2 \mathrm{~mol}\) of \(\mathrm{H}_{2}\)
3) \(2 / 3 \mathrm{~mol}\) of \(\mathrm{O}_{2}\) 4) both \(1 / 2 \mathrm{~mol}\) of \(\mathrm{H}_{2}\) and \(1 / 2 \mathrm{~mol}\) of \(\mathrm{O}_{2}\)
70. Consider a titration of potassium dichromate solution with acidified Mohr's salt solution using diphenylamine as indicator. The number of moles of Mohr's salt required per mole of dichromate is
1) 3
2) 4
3) 5
4) 6

3. CONCEPT: Atomic Structure


Important/Critical Points to Remember
1. The wavelength of limiting line \(=\frac{\mathrm{n}_{1}^{2}}{\mathrm{R}_{\mathrm{H}}}\) for all series. So, for lyman series \(=\frac{1}{\mathrm{R}_{\mathrm{H}}}\)
2. Spectral Series
(i) Lyman Series
Region
(ii) Balmer Series
UV
(iii) Paschen Series
(iv) Brackett Series \(\quad 4\)
(v) Pfund Series \(\quad 5\)
\(\begin{array}{llll}\text { (vi) Humphrey } & 6 & 7,8 \ldots & \text { Far IR }\end{array}\)
3. In terms of time and energy Heisenberg's uncertainty principle may be given as \(\Delta \mathrm{E} \Delta \mathrm{t} \geq \frac{\mathrm{h}}{4 \pi}\) (for energy and time)
4. \((\mathrm{n}+l)\) rule
This rule states that electrons are filled in orbitals according to their increasing values of \(n+\ell\). When \((\mathrm{n}+\ell)\) is same for sub energy levels, the electrons first occupy the sublevels with lowest "n" value. Thus, order of filling up of orbitals is as follows:
\(1 s<2 s<2 p<3 s<3 p<4 s<3 d<4 p<5 s<4 d<5 p<6 s<4 f<5 d\)
5. Orbital \& Spin Angular Momentum
\(\frac{\mathrm{h}}{2 \pi} \sqrt{\ell(\ell+1),} \frac{\mathrm{h}}{2 \pi} \sqrt{\mathrm{s}(\mathrm{s}+1)}\)
\(1 / 2 \mathrm{~m} v^{2}=\mathrm{h} v-\mathrm{h} v^{0}(\mathrm{w})\left(\right.\) work function or B.E.) ; \(\quad v^{0}=\) Threshold frequency \(\mathrm{W}=h v^{0}=\frac{h c}{\lambda_{0}}\)
1. Radii of Orbits
\section{Tips to Problem Solving}
\(r=\frac{\left(4 \pi \varepsilon_{0}\right) n^{2} h^{2}}{4 \pi^{2} \mathrm{mZe}^{2}}\)
By putting value of constants \(r=0.529 \times \frac{n^{2}}{Z} \stackrel{0}{\mathrm{~A}} ; r=0.529 \times \frac{n^{2}}{Z} \times 10^{-10} \mathrm{~m}\);
\(\mathrm{r}=0.529 \times 10^{-8} \times \frac{\mathrm{n}^{2}}{\mathrm{Z}} \mathrm{cm}\)
For H-like atoms. ; Thus \(r_{n}=r_{1} \times n^{2}\)
2. Speed of Electron
\(\mathrm{v}=\frac{2 \pi \mathrm{Ze} \mathrm{e}^{2}}{\left(4 \pi \varepsilon_{0}\right) \mathrm{nh}}=2.188 \times 10^{6} \times \frac{\mathrm{Z}}{\mathrm{n}} \mathrm{m} / \mathrm{sec}\)
\section{The Energy of Electron}
Total energy (E) = K.E. + P.E. \(E_{n}=-\frac{2 \pi^{2} Z^{2} m e^{4}}{\left(4 \pi \epsilon_{0}\right)^{2} n^{2} h^{2}}\)
where \(=\) orbit number
\(E_{n}=E_{1} \times \frac{Z^{2}}{n^{2}}\) for \(H\)-like atoms
\(\mathrm{E}_{\mathrm{n}}=-\frac{21.79 \times 10^{-19} \mathrm{Z}^{2}}{\mathrm{n}^{2}} \mathrm{~J} /\) atom \(=-\frac{13.6}{\mathrm{n}^{2}} \mathrm{Z}^{2}\) eV per atom
\(=-\frac{313.6 \times \mathrm{Z}^{2}}{\mathrm{n}^{2}} \mathrm{kcal} / \mathrm{mol}\)
4. Relation Between Potential energy (P.E), Kinetic energy
(K.E) \& Total energy
T.E. \(=\frac{\text { P.E. }}{2}=-\) K.E.
P.E. \(=-\frac{\mathrm{Ze}^{2}}{\mathrm{r}}\), K.E. \(=\frac{1}{2} \frac{\mathrm{Ze}^{2}}{\mathrm{r}}\), T.E. \(=-\frac{1}{2} \frac{\mathrm{Ze}^{2}}{\mathrm{r}}\)
\section{Rydberg Equation} Rydberg
The wavelength \((\lambda)\), wave number \((\bar{v})\) for the electromagnetic radiation can be calculated by equation.
\(\bar{v}=\frac{1}{\lambda}=\mathrm{R} \times \mathrm{Z}^{2}\left[\frac{1}{\mathrm{n}_{1}^{2}}-\frac{1}{\mathrm{n}_{2}^{2}}\right]\)
\(\mathrm{Z}=\) atomic number
\(\mathrm{R}=109677 \mathrm{~cm}^{-1}-\) Rydberg constant
\(\mathrm{n}_{2}=\) higher orbit
\(\mathrm{n}_{1}=\) lower orbit
5. Total number of spectral lines
(i) \(\frac{\mathrm{n}(\mathrm{n}-1)}{2} \rightarrow\) when electron jumps from nth level to ground level
(ii) \(\frac{\left(\mathrm{n}_{2}-\mathrm{n}_{1}\right)\left(\mathrm{n}_{2}-\mathrm{n}_{1}+1\right)}{2} \rightarrow\) when electron returns from \(\mathrm{n}_{2}\) to \(\mathrm{n}_{1}\).
4. PRACTICE SET: Atomic Structure
\section{LEVEL-1}
TOPIC 1: Discovery of Subatomic Particles, Atomic and Isoelectronic Species
1. The number of neutrons in dipositive zinc ion with mass number 70 is
(1) 34
(2) 36
(3) 38
(4) 40
2. Which of the following pairs are isosters?
(1) \(\mathrm{CO}_{2}\) and \(\mathrm{N}_{2} \mathrm{O}\)
(2) \(\mathrm{CaO}\) and \(\mathrm{KF}\)
(3) \(\mathrm{OF}_{2}\) and \(\mathrm{HClO}\)
(4) All of these
3. What is the optimum conditions required to study the conduction of electricity through gases?
(1) High pressure and low voltage
(2) High pressure and high voltage
(3) Low pressure and high voltage
(4) Low pressure and low voltage
4. In discharge tube experiment stream of negatively charged particles travel from travel
(1) anode to cathode
(2) cathode to anode
(3) Both (1) and (2)
(4) Electrons does not
5. Which one of the following sets of ions represents the collection of isoelectronic species? \(\mathrm{Al}^{3+}, \mathrm{Cl}^{-}\)
(1) \(\mathrm{K}^{+}, \mathrm{Cl}^{-}, \mathrm{Mg}^{2+}, \mathrm{Sc}^{3+}(2) \mathrm{Na}^{+}, \mathrm{Ca}^{2+}, \mathrm{Sc}^{3+}, \mathrm{F}^{-}(3) \mathrm{K}^{+}, \mathrm{Ca}^{2+}, \mathrm{Sc}^{3+}, \mathrm{Cl}^{-}\)
(4) \(\mathrm{Na}^{+}, \mathrm{Mg}^{2+}\)
(Atomic numbers : \(\mathrm{F}=9, \mathrm{Cl}=17, \mathrm{Na}=11, \mathrm{Mg}=12, \mathrm{Al}=13, \mathrm{~K}=19, \mathrm{Ca}=20, \mathrm{Sc}=21\) )
6. Which is correct statement about proton?
(1) Proton is nucleus of deuterium
(2) Proton is a-particle
(3) Proton is ionized hydrogen molecule
(4) Proton is ionized hydrogen atom
7. Which of the following pairs of nucleides are isodiaphers?
(1) \({ }_{6}^{13} \mathrm{C}\) and \({ }_{8}^{16} \mathrm{O}\)
(2) \({ }_{1}^{1} \mathrm{H}\) and \({ }_{1}^{2} \mathrm{H}\)
(3) \({ }_{1}^{3} \mathrm{H}\) and \({ }_{2}^{4} \mathrm{He}\)
(4) \({ }_{25}^{55} \mathrm{Mn}\) and \({ }_{30}^{65} \mathrm{Zn}\)
8. What is the ratio of mass of an electron to the mass of a proton?
(1) \(1: 2\)
(2) \(1: 1\)
(3) \(1: 1837\)
(4) \(1: 3\)
9. Of the following sets which one does NOT contain isoelectronic species?
(1) \(\mathrm{BO}_{3}^{3-}, \mathrm{CO}_{3}^{2-}, \mathrm{NO}_{3}^{-}\)
(2) \(\mathrm{SO}_{3}^{2-}, \mathrm{CO}_{3}^{2-}, \mathrm{NO}_{3}^{-}\)
(3) \(\mathrm{CN}^{-}, \mathrm{N}_{2}, \mathrm{C}_{2}^{2-}\)
(4) \(\mathrm{PO}_{4}^{3-}, \mathrm{SO}_{4}^{2-}, \mathrm{ClO}_{4}^{-}\)
10. In which of the following the amount of deviation from their path in the presence of electric and magnetic field will be maximum?
(1) \(\mathrm{N}^{2-}\)
(2) \(\mathrm{N}^{3-}\)
(3) \(\mathrm{N}^{-}\)
(4) \(\mathrm{N}\)
TOPIC 2: Atomic Models, Emission and Absorption Spectrum
11. The energy of an electron in the \(n^{\text {th }}\) Bohr's orbit of hydrogen atom is
(1) \(-\frac{13.6}{n^{4}} \mathrm{eV}\)
(2) \(-\frac{13.6}{n^{3}} \mathrm{eV}\)
(3) \(-\frac{13.6}{n^{2}} \mathrm{eV}\)
(4) \(-\frac{13.6}{n} \mathrm{eV}\)
12. Which of the following does not contain number of neutrons equal to that of \({ }_{18}^{40} \mathrm{Ar}\) ?
(1) \({ }_{19}^{41} \mathrm{~K}\)
(2) \({ }_{21}^{43} \mathrm{Sc}\)
(3) \({ }_{21}^{40} \mathrm{Sc}\)
(4) \({ }_{20}^{42} \mathrm{Ca}\)
13. When an electron of charge e and mass m moves with a velocity \(\mathrm{v}\) towards the nuclear charge \(\mathrm{Ze}\) in circular orbit of radius \(r\), the potential energy of the electrons is given by
(1) \(\mathrm{Ze}^{2} / \mathrm{r}\)
(2) \(-\mathrm{Ze}^{2} / \mathrm{r}\)
(3) \(\mathrm{Ze}^{2} / \mathrm{r}\)
(4) \(\mathrm{mv}^{2} / \mathrm{r}\)
14. The Bohr's orbit radius for the hydrogen atom \((n=1)\) is approximately \(0.530 \AA\). The radius for the first excited state \((n=2)\) orbit is (in \(\AA\) )
(1) 0.13
(2) 1.06
(3) 4.77
(4) 2.12
15. According to Bohr's theory, the angular momentum of an electron in 5th orbit is
(1) \(10 \mathrm{~h} / \pi\)
(2) \(2.5 \mathrm{~h} / \pi\)
(3) \(25 \mathrm{~h} / \pi\)
(4) \(1.0 \mathrm{~h} / \pi\)
16. An electron from one Bohr stationary orbit can go to next higher orbit
(1) by emission of electromagnetic radiation.
(2) by absorption of any electromagnetic radiation. (3) by absorption of electromagnetic radiation of particular frequency.
(4) without emission or absorption of electromagnetic radiation.
17. The potential energy of electron present in ground state of \(\mathrm{Li}^{2+}\) ion is represented by:
(1) \(\frac{+3 e^{2}}{4 \pi \varepsilon_{0} r}\)
(2) \(\frac{-3 e}{4 \pi \varepsilon_{0} r}\)
(3) \(\frac{-3 e^{2}}{4 \pi \varepsilon_{0} r^{2}}\)
(4) \(\frac{-3 e^{2}}{4 \pi \varepsilon_{0} r}\)
18. The angular speed of the electron in nth orbit of Bohr hydrogen atom is
(1) directly proportional to \(\mathrm{n}\)
(2) inversely proportional of \(\sqrt{\mathrm{n}}\)
(3) inversely proportional to \(\mathrm{n}^{2}\)
(4) inversely proportional to \(\mathrm{n}^{3}\)
19. The radius of 1st Bohr's orbit for hydrogen atom is ' \(r\) '. The radius of second Bohr's orbit is
(1) \(4 \mathrm{r}\)
\((2) r^{3}\)
(3) \(4 r^{2}\)
(4) \(r^{1 / 3}\)
20. Which of the following pairs will have same chemical properties?
(1) \({ }_{6}^{14} \mathrm{C}\) and \({ }_{7}^{15} \mathrm{~N}\)
(2) \(\mathrm{O}^{2-}\) and \(\mathrm{F}^{-}\)
(3) \({ }_{18}^{40} \mathrm{Ar}\) and \({ }_{19}^{40} \mathrm{~K}\)
(4) \({ }_{17}^{35} \mathrm{Cl}\) and \(_{17}^{37} \mathrm{Cl}\)
21. If \(m\) and e are the mass and charge of the revolving electron in the orbit of radius \(r\) for hydrogen atom, the total energy of the revolving electron will be:
(1) \(\frac{1}{2} \frac{\mathrm{e}^{2}}{\mathrm{r}}\)
(2) \(-\frac{\mathrm{e}^{2}}{\mathrm{r}}\)
(3) \(\frac{m e^{2}}{\mathrm{r}}\)
(4) \(-\frac{1}{2} \frac{e^{2}}{\mathrm{r}}\)
22. Energy of an electron in a one-electron system can be calculated as
\(\mathrm{E}_{\mathrm{n}}=\frac{-2.18 \times 10^{-18} \mathrm{Z}^{2}}{\mathrm{n}^{2}}\)
Which of the following correctly states the relationship between the \(n=2\) level of He+ atom \((Z\) \(=2\) ) and \(n=2\) level of \(\mathrm{Li}^{2+}\) ion \((Z=3)\) ?
(1) \(\mathrm{E}_{\mathrm{He}^{+}}=\frac{9}{4} \mathrm{E}_{\mathrm{Li}^{2+}}\)
(2) \(\mathrm{E}_{\mathrm{He}^{+}}=\frac{4}{9} \mathrm{E}_{\mathrm{Li}^{2+}}\)
(3) \(\mathrm{E}_{\mathrm{He}^{+}}=\frac{9}{2} \mathrm{E}_{\mathrm{Li}^{2+}}\)
(4) \(\mathrm{E}_{\mathrm{He}^{+}}=\frac{2}{9} \mathrm{E}_{\mathrm{Li}^{2+}}\)
23. What is the difference between two species if one has atomic mass \(=14\) and atomic number \(=\) 7 whereas the other has atomic mass \(=14\) and atomic number \(=6\) ?
(1) Neutrons
(2) Protons
(3) Electrons
(4) All of these
24. The velocity of an electron in excited state of \(\mathrm{H}\)-atom is \(1.093 \times 10^{6} \mathrm{~m} / \mathrm{s}\). What is the circumference of this orbit?
(1) \(3.32 \times 10^{-10} \mathrm{~m}\)
(2) \(6.64 \times 10^{-10} \mathrm{~m}\)
(3) \(13.30 \times 10^{-10} \mathrm{~m}\)
(4) \(13.28 \times 10^{-8} \mathrm{~m}\)
25. Monochromatic radiation of specific wavelength is incident on \(\mathrm{H}\)-atoms in ground state. \(\mathrm{H}-\) atoms absorb energy and emit subsequently radiations of six different wavelength. Find wavelength of incident radiations:
(1) \(9.75 \mathrm{~nm}\)
(2) \(50 \mathrm{~nm}\)
(3) \(85.8 \mathrm{~nm}\)
(4) \(97.25 \mathrm{~nm}\)
26. If I excitation energy for the \(\mathrm{H}\)-like (hypothetical) sample is \(24 \mathrm{eV}\), then binding energy in III excited state is:
(1) \(2 \mathrm{eV}\)
(2) \(3 \mathrm{eV}\)
(3) \(4 \mathrm{eV}\)
(4) \(5 \mathrm{eV}\)
27. The energy of separation of an electron in a hydrogen like atom in excited state is \(3.4 \mathrm{eV}\). The de-Broglie wave length (in \(\AA\) ) associated with the electron is: (Given radius of first orbit of \(\mathrm{H}\) atom is \(0.53 \AA)\)
(1) 3.33
(2) 6.66
(3) 13.31
(4) None of these
28. If an electron undergoes transition from \(\mathrm{n}=2\) to \(\mathrm{n}=1\) in \(\mathrm{Li}^{2+}\) ion, the energy of photon radiated will be best given by
(1) \(\mathrm{h} v\)
(2) h \(v_{1}+\mathrm{h} v_{2}\)
(3) \(h v_{1}+h v_{2}+h v_{3}\)
(4) All of these 29. The photoelectric current decreases if
(1) the intensity of the source of light is decreased.
(2) the frequency of incident radiation decreases below threshold frequency.
(3) the exposure time decreases.
(4) none of these.
30. What will be the difference between electromagnetic radiation shown in A and B respectively?


(i) Velocity
(ii) Wavelength
(1) (ii) only
(2) (ii) and (iv)
(iii) Frequency
(iv) Energy
(3) (ii), (iii) and (iv)
(4) (iv) only
31. The work function of a photoelectric material is \(3.3 \mathrm{eV}\), its threshold frequency will be
(1) \(8 \times 10^{14} \mathrm{~Hz}\)
(2) \(5 \times 10^{33} \mathrm{~Hz}\)
(3) \(8 \times 10^{10} \mathrm{~Hz}\)
(4) \(4 \times 10^{11} \mathrm{~Hz}\)
32. Arrange the electromagnetic radiations a, b, c, \(d\) and e in increasing order of energy.
Frequencies of a, b and c are \(10^{15}, 10^{14}\) and \(10^{17}\) respectively whereas wavelength of (d) and (e) are \(350 \mathrm{~nm}\) and \(100 \mathrm{~nm}\) respectively?
(1) a, b, c, d, e
(2) a, b, d, e, c
(3) a, d, b, e, c
(4) b, d, a, e, c
33. For any \(\mathrm{H}\) like system, the ratio of velocities of electron in I, II and III orbit i.e., \(\mathrm{v}_{1}: \mathrm{v}_{2}: \mathrm{v}_{3}\) will be :
(1) \(1: 2: 3\)
(2) \(1: 1 / 2: 1 / 3\)
(3) \(3: 2: 1\)
(4) \(1: 1: 1\)
34. In which of the following Bohr's stationary state, the electron will be at maximum distance from the nucleus?
(1) IInd
(2) Ist
(3) Vth
(4) IIIrd
35. What is the potential energy of an electron present in \(\mathrm{N}\)-shell of the Be ion?
(1) \(-3.4 \mathrm{eV}\)
(2) \(-6.8 \mathrm{eV}\)
(3) \(-13.6 \mathrm{eV}\)
(4) \(-27.2 \mathrm{eV}\)
36. Which of the following transitions of electrons in the hydrogen atom will emit maximum energy?
(1) \(n_{5} \rightarrow n_{4}\)
(2) \(n_{4} \rightarrow n_{3}\)
(3) \(n_{3} \rightarrow n_{2}\)
(4) all will emit same
energy
37. What is the ratio of time periods \(\left(\mathrm{T}_{1} / \mathrm{T}_{2}\right)\) in second orbit of hydrogen atom to third orbit of \(\mathrm{He}^{+}\)ion?
(1) \(8 / 27\)
(2) \(32 / 27\)
(3) \(27 / 32\)
(4) None of these
38. Bohr's model is not able to account for which of the following.
(1) Stability of atom.
(2) Spectrum of neutral helium atom.
(3) Energy of free electron at rest.
(4) Calculation of radii of the stationary states.
TOPIC 3: Planck's Quantum Theory, Heisenberg's Uncertainty Principle and Schrodinger's Wave Equation:
39. If the energy of a photon is \(3.03 \times 10^{-19} \mathrm{~J}\) then, the wavelength \((\lambda)\) of the photon is :
(1) \(6.56 \mathrm{~nm}\)
(2) \(65.6 \mathrm{~nm}\)
(3) \(656 \mathrm{~nm}\)
(4) \(0.656 \mathrm{~nm}\)
40. The value of Planck's constant is \(6.63 \times 10^{-34} \mathrm{Js}\). The velocity of light is \(3.0 \times 10^{8} \mathrm{~ms}^{-1}\). Which value is closest to the wavelength in nanometers of a quantum of light with frequency of \(8 \times\) \(10^{15} \mathrm{~s}^{-1}\) ?
(1) \(3 \times 10^{7}\)
(2) \(2 \times 10^{-25}\)
(3) \(5 \times 10^{-18}\)
(4) \(4 \times 10^{1}\)
41. The de Broglie wavelength of a tennis ball of mass \(60 \mathrm{~g}\) moving with a velocity of 10 metres per second is approximately Planck's constant, \(\mathrm{h}=6.63 \times 10^{-34} \mathrm{Js}\)
(1) \(10^{-31}\) metres
(2) \(10^{-16}\) metres
(3) \(10^{-25}\) metres
(4) \(10^{-33}\) metres
42. Wavelength associated with electron motion
(1) increases with increase in speed of electron.
(2) remains same irrespective of speed of electron.
(3) decreases with increase of speed of \(\mathrm{e}^{-}\)(electron).
(4) is zero.
43. The Heisenberg uncertainty principle will be most significant for which of the following object?
(1) Object A of mass \(9.11 \times 10^{-30} \mathrm{~kg}\)
(2) Object B of mass \(9.11 \times 10^{-28} \mathrm{~g}\)
(3) Object C of mass \(9.11 \times 10^{-24} \mathrm{mg}\)
(4) Object D of mass \(9.11 \times 10^{-28} \mathrm{~kg}\)
44. The momentum (in \(\mathrm{kg}-\mathrm{m} / \mathrm{s}\) ) of photon having \(6 \mathrm{MeV}\) energy is :
(1) \(3.2 \times 10^{-21}\)
(2) 2.0
(3) \(1.6 \times 10^{-21}\)
(4) None of these
45. When electronic transition occurs from higher energy state to lower energy state with energy difference equal to \(\Delta \mathrm{E}\) electron volts, the wavelength of the line emitted is approximately equal to
(1) \(\frac{12395}{\Delta \mathrm{E}} \times 10^{-10} \mathrm{~m}\)
(2) \(\frac{12395}{\Delta \mathrm{E}} \times 10^{10} \mathrm{~m}\)
(3) \(\frac{12395}{\Delta \mathrm{E}} \times 10^{-10} \mathrm{~cm}\)
(4) \(\frac{12395}{\Delta \mathrm{E}} \times 10^{10} \mathrm{~cm}\)
46. Which of the following statement concerning probability density \(\left(\psi^{2}\right)\) and radial distribution function
\(\left(4 \pi r^{2} \psi^{2}\right)\) for a s-orbital of H-like species is correct?
(1) \(\psi^{2}\) is minimum at nucleus but \(4 \pi r^{2} \psi^{2}\) is maximum at nucleus.
(2) \(\psi^{2}\) is maximum at nucleus but \(4 \pi r^{2} \psi^{2}\) is minimum at nucleus.
(3) Both \(\psi^{2}\) and \(4 \pi r^{2} \psi^{2}\) are maximum at nucleus. (4) Both \(\psi^{2}\) and \(4 \pi r^{2} \psi^{2}\) are minimum at nucleus.
47. The angular momentum of \(d\) electron is
(1) \(\frac{h}{2 \pi} \sqrt{6}\)
(2) \(\frac{h}{\pi} \sqrt{6}\)
(3) \(\frac{\mathrm{h}}{2 \pi} \sqrt{2}\)
(4) \(\frac{h}{\pi} \sqrt{2}\)
48. If \(E_{1}, E_{2}\), and \(E_{3}\) represent respectively the kinetic energies of an electron and an alpha particle and a proton each having same de-Broglie wavelength then
(1) \(\mathrm{E}_{1}>\mathrm{E}_{3}>\mathrm{E}_{2}\)
(2) \(\mathrm{E}_{2}>\mathrm{E}_{3}>\mathrm{E}_{1}\)
(3) \(\mathrm{E}_{1}>\mathrm{E}_{2}>\mathrm{E}_{3}\)
(4) \(\mathrm{E}_{1}=\mathrm{E}_{2}=\mathrm{E}_{3}\)
49. If uncertainty in position and momentum are equal, then uncertainty in velocity is :
(1) \(\frac{1}{2 m} \sqrt{\frac{h}{\pi}}\)
(2) \(\sqrt{\frac{\mathrm{h}}{2 \pi}}\)
(3) \(\frac{1}{\mathrm{~m}} \sqrt{\frac{\mathrm{h}}{\pi}}\)
(4) \(\sqrt{\frac{h}{\pi}}\)
50. Which of the following statement is wrong about photon ?
(1) Photon's energy is \(h v\).
(3) Momentum of photon is \(\frac{h v}{\mathrm{c}}\)
(4) Photon exerts no pressure.
(2) Photon's rest mass is zero.
51. Excited hydrogen atom emits light in the ultraviolet region at \(2.47 \times 10^{15} \mathrm{~Hz}\). With this frequency, the energy of a single photon is: \(\left(\mathrm{h}=6.63 \times 10^{-34} \mathrm{Js}\right)\)
(1) \(8.041 \times 10^{-40} \mathrm{~J}\)
(2) \(2.680 \times 10^{-19} \mathrm{~J}\)
(3) \(1.640 \times 10^{-18} \mathrm{~J}\)
(4) \(6.111 \times 10^{-17} \mathrm{~J}\)
52. Ionization energy of gaseous \(\mathrm{Na}\) atoms is \(495.5 \mathrm{~kJ} \mathrm{~mol}^{-1}\). The lowest possible frequency of light that
ionizes a sodium atom is \(\left(\mathrm{h}=6.626 \times 10^{-34} \mathrm{Js}, \mathrm{N}_{\mathrm{A}}=6.022 \times 10^{23} \mathrm{~mol}^{-1}\right)\)
(1) \(7.50 \times 10^{4} \mathrm{~s}^{-1}\)
(2) \(4.76 \times 10^{14} \mathrm{~s}^{-1}\)
(3) \(3.15 \times 10^{15} \mathrm{~s}^{-1}\)
(4) \(1.24 \times 10^{15} \mathrm{~s}^{-1}\) TOPIC 4: Quantum Numbers, Electronic Configuration and Shape of Orbitals
53. Which one of the following set of quantum numbers is not possible for \(4 \mathrm{p}\) electron?
(1) \(\mathrm{n}=4, \ell=1, \mathrm{~m}=-1, \mathrm{~m}_{\mathrm{s}}=+\frac{1}{2}\)
(2) \(\mathrm{n}=4, \ell=1, \mathrm{~m}=0, \mathrm{~m}_{\mathrm{s}}=+\frac{1}{2}\)
(3) \(\mathrm{n}=4, \ell=1, \mathrm{~m}=2, \mathrm{~m}_{\mathrm{s}}=+\frac{1}{2}\)
(4) \(\mathrm{n}=4, \ell=1, \mathrm{~m}=-1, \mathrm{~m}_{\mathrm{s}}=-\frac{1}{2}\)
54. What is the correct orbital designation of an electron with the quantum number, \(n=4, l=3, m\) \(=-2\) \(s=1 / 2 ?\)
(1) \(3 s\)
(2) \(4 f\)
(3) \(5 p\)
(4) \(6 s\)
55. Which orbital of the following is lower in energy in a many electron atom?
(1) \(2 s\)
(2) \(3 d\)
(3) \(4 s\)
(4) \(5 f\)
56. Which of the following graph correspond to one node
- e



57. The total number of electrons that can be accommodated in all orbitals having principal quantum number 2 and azimuthal quantum number 1 is
(1) 2
(2) 4
(3) 6
(4) 8
58. What can be the representation of the orbital having 3 angular nodes and \(n=5\).
(1) \(5 d\)
(2) \(5 f\)
(3) \(5 p\)
(4) \(5 \mathrm{~s}\)
59. The five \(d\)-orbitals are designated as \(d_{x y}, d_{y z}, d_{x z}, d_{x^{2}-y^{2}}\) and \(d_{z^{2}}\). Choose the correct statement.
(1) The shapes of the first three orbitals are similar but that of the fourth and fifth orbitals are different.
(2) The shapes of all five d-orbitals are similar.
(3) The shapes of the first four orbitals are similar but that of the fifth orbital is different.
(4) The shapes of all five d-orbitals are different.
60. Maximum number of electrons in a subshell of an atom is determined by the following:
(1) \(2 l+1\)
(2) \(4 l-2\)
(3) \(2 n^{2}\)
(4) \(4 l+2\)
61. An e- has magnetic quantum number as -3 , what is its principal quantum number?
(1) 1
(2) 2
(3) 3
(4) 4
62. For \(a, f\)-orbital, the values of \(m\) are
(1) \(-2,-1,0,+1,+2\)
(2) \(-3,-2,-1,0,+1,+2,+3\)
(3) \(-1,0,+1\)
(4) \(0,+1,+2,+3\)
63. A \(5 f\) orbital has
(1) one node
(2) two nodes
(3) three nodes
(4) four nodes.
64. If electron has spin quantum number \(+1 / 2\) and a magnetic quantum number -1 , it cannot be present in
(1) \(d\)-orbital
(2) f-orbital
(3) \(p\)-orbital
(4) s-orbital.
65. The orbital angular momentum for an electron revolving in an orbit is given by \(\sqrt{l(l+1)} \cdot \frac{h}{2 \pi}\). This momentum for an s-electron will be given by
(1) zero
(2) \(\frac{h}{2 \pi}\)
(3) \(\sqrt{2} \cdot \frac{h}{2 \pi}\)
(4) \(+\frac{1}{2} \cdot \frac{h}{2 \pi}\)
66. The energy of the electron in \(\mathrm{Be}^{3+}\) ion depends on
(1) the principal quantum number only.
(2) the principal and azimuthal quantum numbers only.
(3) the principal, azimuthal and magnetic quantum numbers only.
(4) the principal, azimuthal, magnetic and spin quantum numbers.
67. What are the component values (in terms of \(h / 2 \pi\) ) of the orbital angular momentum along the Z-direction for a \(2 p\) electron?
(1) \(+\frac{1}{2},-\frac{1}{2}\)
(2) \(+\frac{3}{2},+\frac{1}{2},-\frac{1}{2},-\frac{3}{2}\)
(3) \(+2,+1,0,-1,-2\)
\((4)+1,0,-1\)
68. The total number of orbitals associated with the principal quantum number 5 is :
(1) 20
(2) 25
(3) 10
(4) 5
69. The total spin and magnetic moment for the atom with atomic number 24 are:
(1) \(\pm 3, \sqrt{48} \mathrm{BM}\)
(2) \(\pm 3, \sqrt{35} \mathrm{BM}\)
(3) \(\pm \frac{3}{2}, \sqrt{48} \mathrm{BM}\)
(4) \(\pm \frac{3}{2}, \sqrt{35} \mathrm{BM}\)
70. A principal shell having the highest energy subshell to be ' \(g\) ' can accomodate electrons to a maximum of
(1) 18
(2) 32
(3) 25
(4) 50
5. CONCEPT: CHEMICAL BONDING


6. THEORY-CHEMICAL BONDING
Important Points to Remember
There are two types of \(\mathrm{H}\)-boding (i) Intermolecular \(\mathrm{H}\)-bonding (association).
\(\mathrm{H}\)-bonding involving two or more molecules.
(ii) Intramolecular \(\mathrm{H}\)-bonding (chelation). \(\mathrm{H}\)-bonding taking place within single molecule.
Applications of Intermolecular H-Bonding
(i) Water: Water has the lowest molecular weight among the hydrides of group 16 elements yet it has the highest melting and boiling points.
(ii) Ice has less density than water
Amides associate and have higher melting and boiling points.
Applications of Intramolecular H-Bonding
(i) Salicylic acid is stronger acid than \(o\)-methoxy benzoic acid
(ii) Ethyl aceto acetate - It exists in two forms

Keto form
Enolic form is more volatile due to chelation

(iii) Maleic acid is stronger acid than fumaric acid
Maleate ion can be stabilised by Chelation because hydrogen and oxygen responsible for forming hydrogen bond are very near to each other. On the other hand fumerate ion cannot stablise by chelation because hydrogen and oxygen are on opposite sides to each other. Hence formation of fumerate ion does not take place.
(iv) Acid character of nitrophenols. It follows the following order \(p\) - nitrophenol \(>0\) - nitrophenol \(>m\) - nitrophenol Acid character of o - nitrophenol is suppressed by chelation

(v) o-nitrophenol is more volatile (b.pt \(214^{\circ} \mathrm{C}\) ) as compared to meta (b.pt \(290^{\circ} \mathrm{C}\) ) and para (b.pt \(279^{\circ} \mathrm{C}\) ). It is due to chelation
b) \(\Delta \mathrm{H}_{\mathrm{f}}=\Delta \mathrm{H}_{\mathrm{sub}}+\frac{1}{2} \mathrm{DH}_{\mathrm{d}}+\mathrm{I} \cdot \mathrm{E} .+\mathrm{E} \cdot \mathrm{A} \cdot+\mathrm{U}\)
where,
\(\left.\begin{array}{l}\Delta \mathrm{H}_{\text {sub }}=\text { Enthalpy of formation } \\ \Delta \mathrm{H}_{\text {sul }}=\text { Enthalpy of sublimation } \\ \Delta \mathrm{H}_{\mathrm{d}}=\text { Enthalpy of dissociation } \\ \text { I.E. = Ionization energy } \\ \text { E.A. = Electron affinity } \\ \mathrm{U}=\text { Lattice energy or Lattice enthalpy }\end{array}\right\}\) positive
In order to determine the number of \(p p-p p\) or \(p p-a p\) bonds, the following formula can be used. (i) Calculate the number of \(s\) bonds = number of surrounding atoms
(ii) \(\mathrm{p}\) bonds = number of oxygen atoms - number of negative charge
(iii) Lone pair \(=1 / 2\) (Valence electron - number of covalent bond)
(iv) Hybridization \(=s\) bonds + number of lone pairs
(v) Now, number of \(p p-p p\) bonds = number of unhybridized \(p\)-orbitals left
(vi) \(p p-d\) p bonds will be formed when \(p\) bonds are more than the number of unhybridized \(p\)-orbitals left.
The more the electronegativity of atom involved in \(\mathrm{H}\)-bonding, the more is the bond strength eg. \(\mathrm{HLF} \quad>\mathrm{HLO} \quad>\mathrm{HLN}\)
\(10 \mathrm{kcal} / \mathrm{mole} \quad>7 \mathrm{kcal} / \mathrm{mole} \quad>2.0 \mathrm{kcal} / \mathrm{mole}\)
7. EXERCISE-1 CHEMICAL BONDING
\section{LEVEL-1 \\ TOPIC 1: Electrovalent, Covalent and Coordinate Bonding}
1. Which of the following combination will form an electrovalent bond ?
1) \(\mathrm{P}\) and \(\mathrm{Cl}\)
2) \(\mathrm{NH}_{3}\) and \(\mathrm{BF}_{3}\)
3) \(\mathrm{H}\) and \(\mathrm{Ca}\)
4) \(\mathrm{H}\) and \(\mathrm{S}\)
2. Which has a giant covalent structure?
1) \(\mathrm{PbO}_{2}\)
2) \(\mathrm{SiO}_{2}\)
3) \(\mathrm{NaCl}\)
4) \(\mathrm{AlCl}_{3}\)
3. Which one of the following contains a co-ordinate covalent bond ?
1) \(\mathrm{H}_{2} \mathrm{O}\)
2) \(\mathrm{HCl}\)
3) \(\mathrm{BaCl}_{2}\)
4) \(\mathrm{N}_{2} \mathrm{H}_{5}^{+}\)
4. The number of dative bonds in sulphuric acid molecule is
1) 0
2) 1
3) 2
4) 4
5. Which of the following statements is not true about covalent compounds?
1) They may exhibit space isomerism
2) They have low melting and boiling points
3) They show ionic reactions
4) They show molecular reactions
6. Indicate the nature of bonding in \(\mathrm{CCl}_{4}\) and \(\mathrm{CaH}_{2}\)
1) Covalent in \(\mathrm{CCl}_{4}\) and electrovalent in \(\mathrm{CaH}_{2}\)
2) Electrovalent in both \(\mathrm{CCl}_{4}\) and \(\mathrm{CaH}_{2}\)
3) Covalent in both \(\mathrm{CCl}_{4}\) and \(\mathrm{CaH}_{2}\)
4) Electrovalent in \(\mathrm{CCl}_{4}\) and covalent in \(\mathrm{CaH}_{2}\)
7. Lattice energy of an ionic compound depends upon
1) charge on the ion and size of the ion
2) packing of ions only
3) size of the ion only
4) charge on the ion only
8. Among the following which compound will show the highest lattice energy ?
1) \(\mathrm{KF}\)
2) \(\mathrm{NaF}\)
3) \(\operatorname{CsF}\)
4) \(\mathrm{RbF}\)
9. The compound that has the highest ionic character associated with the \(\mathrm{X}-\mathrm{Cl}\) bond is:
1) \(\mathrm{PCl}_{5}\)
2) \(\mathrm{BCl}_{3}\)
3) \(\mathrm{CCl}_{4}\)
4) \(\mathrm{SiCl}_{4}\)
10. Which combination of atoms can form a polar covalent bond?
1) \(\mathrm{H}\) and \(\mathrm{H}\)
2) \(\mathrm{H}\) and \(\mathrm{F}\)
3) \(\mathrm{N}\) and \(\mathrm{N}\)
4) Na and \(F\)
11. Which of the following pairs will form the most stable ionic bond ?
1) \(\mathrm{Na}\) and \(\mathrm{Cl}\)
2) \(\mathrm{Mg}\) and \(\mathrm{F}\)
3) \(\mathrm{Li}\) and \(\mathrm{F}\)
4) \(\mathrm{Na}\) and \(\mathrm{F}\)
12. In which of the following species central atom is NOT surrounded by exactly 8 valence electrons?
1) \(\mathrm{BF}_{4}^{-}\)
2) \(\mathrm{NCl}_{3}\)
3) \(\mathrm{PCl}_{4}^{+}\)
4) \(\mathrm{SF}_{4}\)
13. Which of the following does not apply to metallic bond ?
1) Overlapping valence orbitals
2) Mobile valency electrons
3) Delocalized electrons
4) Highly directed bonds.
14. Which set contains only covalently bonded molecules?
1) \(\mathrm{BCl}_{3}, \mathrm{SiCl}_{4}, \mathrm{PCl}_{3}\)
2) \(\mathrm{NH}_{4} \mathrm{Br}, \mathrm{N}_{2} \mathrm{H}_{4}, \mathrm{HBr}\)
3) \(\mathrm{I}_{2}, \mathrm{H}_{2} \mathrm{~S}, \mathrm{NaI}\)
4) \(\mathrm{Al}, \mathrm{O}_{3}, \mathrm{As}_{4}\)
15. Amongst \(\mathrm{LiCl}, \mathrm{RbCl}, \mathrm{BeCl}_{2}\) and \(\mathrm{MgCl}_{2}\) the compounds with the greatest and the least ionic character, respectively are:
1) \(\mathrm{LiCl}\) and \(\mathrm{RbCl}\)
2) \(\mathrm{RbCl}\) and \(\mathrm{BeCl}_{2}\)
3) \(\mathrm{MgCl}_{2}\) and \(\mathrm{BeCl}_{2}\)
4) \(\mathrm{RbCl}\) and \(\mathrm{MgCl}_{2}\)
16. In ionic solids how crystal structure get stabilized
1) By the energy released in the formation of crystal lattice.
2) By achieving octet of electrons around the ionic species in gaseous state.
3) By electron gain enthalpy and the ionization enthalpy.
4) None of these
17. Which of the following statement is correct?
1) \(\mathrm{FeCl}_{2}\) is more covalent than \(\mathrm{FeCl}_{3}\).
2) \(\mathrm{FeCl}_{3}\) is more covalent than \(\mathrm{FeCl}_{2}\).
3) Both \(\mathrm{FeCl}_{2}\) and \(\mathrm{FeCl}_{3}\) are equally covalent. 4) \(\mathrm{FeCl}_{2}\) and \(\mathrm{FeCl}_{3}\) do not have any covalent character. 18. A pair of compounds which has odd electrons in the group \(\mathrm{NO}, \mathrm{CO}, \mathrm{ClO}_{2}, \mathrm{~N}_{2} \mathrm{O}_{5}, \mathrm{SO}_{2}\) and \(\mathrm{O}_{3}\) are
1) \(\mathrm{NO}\) and \(\mathrm{ClO}_{2}\)
2) \(\mathrm{CO}\) and \(\mathrm{SO}_{2}\)
3) \(\mathrm{ClO}_{2}\) and \(\mathrm{CO}\)
4) \(\mathrm{SO}_{2}\) and \(\mathrm{O}_{3}\)
19. Which of the following molecule(s) obey the octet rule?
(i) \(\left[\mathrm{BF}_{4}\right]^{-}\), (ii) \(\left[\mathrm{AlCl}_{4}\right]^{-}\), (iii) \(\mathrm{SO}_{2}\), (iv) \(\mathrm{CCl}_{4}\)
1) (i), (ii), (iii), (iv)
2) (ii), (iii), (iv)
3) (i), (iii), (iv)
4) (i), (ii), (iv)
20. In the cyanide ion, the formal negative charge is on
1) \(\mathrm{C}\)
2) \(\mathrm{N}\)
3) Both \(C\) and \(N\) 4) resonate between \(C\) and \(N\)
21. Among the following, the species having the smallest bond order is
1) \(\mathrm{NO}^{-}\)
2) \(\mathrm{NO}^{+}\)
3) \(\mathrm{O}_{2}\)
4) \(\mathrm{NO}\)
22. The bond length of \(\mathrm{C}=\mathrm{O}\) bond in \(\mathrm{CO}\) is \(1.20 \AA\) and in \(\mathrm{CO}_{2}\) it is \(1.34 \AA\). Then \(\mathrm{C}=\mathrm{O}\) bond length in \(\mathrm{CO}_{3}^{2-}\) will be
1) \(1.50 \AA\)
2) \(1.34 \AA\)
3) \(1.29 \AA\)
4) \(0.95 \AA\)
23. Which one of the following pairs of molecules will have permanent dipole moments for both members ?
1) \(\mathrm{NO}_{2}\) and \(\mathrm{CO}_{2}\)
2) \(\mathrm{NO}_{2}\) and \(\left.\mathrm{O}_{3} 3\right) \mathrm{SiF}_{4}\) and \(\mathrm{CO}_{2}\)
4) \(\mathrm{SiF}_{4}\) and \(\mathrm{NO}_{2}\)
24. Which of the following structure represents structure of \(\mathrm{O}_{3}\) more accurately?

III
1) I and III only
2) II and III only
3) I and II only
4) All
25. Which of the following salt shows maximum covalent character?
1) \(\mathrm{AlCl}_{3}\)
2) \(\mathrm{MgCl}_{2}\)
3) \(\mathrm{CsCl}\)
4) \(\mathrm{LaCl}_{3}\)
26. Pauling's electronegativity values for elements are useful in predicting :
1) polarity of bonds in molecules
2) ionic and covalent nature of bonds
3) coordination number
4) both 1) and 2)
27. The molecule which has zero dipole moment is
1) \(\mathrm{CH}_{3} \mathrm{Cl}\)
2) \(\mathrm{NF}_{3}\)
3) \(\mathrm{BF}_{3}\)
4) \(\mathrm{ClO}_{2}\)
28. Which bond angle q would result in the maximum dipole moment for the triatomic molecule YXY
1) \(q=90^{\circ}\)
2) \(q=120^{\circ}\)
3) \(q=150^{\circ}\)
4) \(q=180^{\circ}\)
29. Polarisibility of halide ions increases in the order
1) \(\mathrm{F}^{-}, \mathrm{I}^{-}, \mathrm{Br}^{-}, \mathrm{Cl}^{-}\)
2) \(\mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-}, \mathrm{F}^{-}\)
3) \(\mathrm{I}^{-}, \mathrm{Br}^{-}, \mathrm{Cl}^{-}, \mathrm{F}^{-}\)
4) \(\mathrm{F}^{-}, \mathrm{Cl}^{-}, \mathrm{Br}^{-}, \mathrm{I}^{-}\)
30. If one assumes linear structure instead of bent structure for water, then which one of the following properties cannot be explained?
1) The formation of intermolecular hydrogen bond in water.
2) The high boiling point of water.
3) Solubility of polar compounds in water.
4) Ability of water to form coordinate covalent bond.
\section{TOPIC 3: VSEPR Theory, VBT Theory and Hybridization}
31. The angle between the overlapping of one s-orbital and one \(p\)-orbital is
1) \(180^{\circ}\)
2) \(120^{\circ}\)
3) \(109^{\circ} 28^{\prime}\)
4) \(120^{\circ} 60^{\prime}\)
32. Equilateral shape has
1) \(s p\) hybridisation
2) \(s p^{2}\) hybridisation
3) \(s p^{3}\) hybridisation
4) None of these
33. Which one of the following has the shortest carbon-carbon bond length ?
1) Benzene
2) Ethene
3) Ethyne
4) Ethane
34. Which of the following is the correct increasing order of lone pair of electrons on the central atom?
1) \(\mathrm{IF}_{7}<\mathrm{IF}_{5}<\mathrm{CIF}_{3}<\mathrm{XeF}_{2}\)
2) \(\mathrm{IF}_{7}<\mathrm{XeF}_{2}<\mathrm{CIF}_{2}<\mathrm{IF}_{5}\)
3) \(\mathrm{IF}_{7}<\mathrm{CIF}_{3}<\mathrm{XeF}_{2}<\mathrm{IF}_{5}\)
4) \(\mathrm{IF}_{7}<\mathrm{XeF}_{2}<\mathrm{IF}_{5}<\mathrm{CIF}_{3}\)
35. In which one of the following molecules the central atom is said to adopt \(s p^{2}\) hybridization?
1) \(\mathrm{BeF}_{2}\)
2) \(\mathrm{BF}_{3}\)
3) \(\mathrm{C}_{2} \mathrm{H}_{2}\)
4) \(\mathrm{NH}_{3}\)
36. Which of the following two are isostructural?
1) \(\mathrm{NH}_{3}, \mathrm{BF}_{3}\)
2) \(\mathrm{PCl}_{5}, \mathrm{ICl}_{5}\)
3) \(\mathrm{XeF}_{2}, \mathrm{IF}_{2}^{-}\)
4) \(\mathrm{CO}_{3}^{-2}, \mathrm{SO}_{3}^{2-}\)
37. The decreasing values of bond angles from NH3 \(\left(106^{\circ}\right)\) to \(\mathrm{SbH} 3\left(101^{\circ}\right)\) down group-15 of the periodic table is due to
1) decreasing \(b p-b p\) repulsion
2) decreasing electronegativity
3) increasing \(b p-b p\) repulsion
4) increasing \(l p\) - \(b p\) repulsion
38. The shape of \(\mathrm{ClO}_{3}\) - ion according to Valence Shell Electron Pair Repulsion (VSEPR) theory will be
1) planar triangular
2) pyramidal
3) tetrahedral
4) square planar
39. Which of the following molecules has trigonal planar geometry?
1) \(\mathrm{BF}_{3}\)
2) \(\mathrm{NH}_{3}\)
3) \(\mathrm{PCl}_{3}\)
4) \(\mathrm{IF}_{3}\)
40. Linear combination of two hybridized orbitals belonging to two atoms and each having one electron leads to a
1) sigma bond
2) double bond
3) co-ordinate covalent bond
4) pi bond.
41. Which of the following statements is not correct for sigma and pi-bonds formed between two carbon atoms?
1) Sigma-bond determines the direction between carbon atoms but a pi-bond has no primary effect in this regard
2) Sigma-bond is stronger than a pi-bond
3) Bond energies of sigma- and pi-bonds are of the order of \(264 \mathrm{~kJ} / \mathrm{mol}\) and \(347 \mathrm{~kJ} / \mathrm{mol}\), respectively
4) Free rotation of atoms about a sigma-bond is allowed but not in case of a pi-bond
42. How many \(s\) and \(p\) bonds are present in toluene?
1) \(3 \pi+8 \sigma\)
2) \(3 \pi+10 \sigma\)
3) \(3 \pi+15 \sigma\)
4) \(6 \pi+3 \sigma\)
43. The number of lone pair and bond pair of electrons on the sulphur atom in sulphur dioxide molecule are respectively
1) 1 and 3
2) 4 and 1
3) 3 and 1
4) 1 and 2
44. How many sigma bonds are in a molecule of diethyl ether, \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OC}_{2} \mathrm{H}_{5}\)
1) 14
2) 12
3) 8
4) 16
45. Which of the following statements is not correct?
1) Hybridisation is the mixing of atomic orbitals prior to their combining into molecular orbitals
2) \(s p^{2}\) hybrid orbitals are formed from two \(p\)-atomic orbitals and one \(s\)-orbital
3) \(d^{2} s p^{3}\) hybrid orbitals are directed towards the corners of a regular octahedron
4) \(d s p^{3}\) hybrid orbitals are all at \(90^{\circ}\) to one another
46. Which of the following species has a linear shape ?
1) \(\mathrm{SO}_{2}\)
2) \(\mathrm{NO}_{2}^{+}\)
3) \(\mathrm{CH}_{4}\)
4) \(\mathrm{NO}_{2}^{-}\)
47. Using VSEPR theory, predict the species which has square pyramidal shape
1) \(\mathrm{SnCl}_{2}\)
2) \(\mathrm{CCl}_{4}\)
3) \(\mathrm{SO}_{3}\)
4) \(\mathrm{BrF}_{5}\)
48. Amongst the following, the molecule/ion that is linear is :
1) \(\mathrm{SO}_{2}\)
2) \(\mathrm{CO}_{2}\)
3) \(\mathrm{ClO}_{2}^{-}\)
4) \(\mathrm{NO}_{2}^{-}\)
49. Which of the following structure is most stable ?

Choose the correct option.
1) Only I
2) Only II
3) Only III
4) All three have same stability
50. The true statements from the following are 1. \(\mathrm{PH}_{5}\) and \(\mathrm{BiCl}_{5}\) do not exist
3. Electrons travel with the speed of light 5. \(\mathrm{I}_{3}^{+}\)has bent geometry
1) 1,3
2) \(1,2,5\)
3) \(1,3,5\)
4) \(1,2,4\)
51. The hybrid state of \(\mathrm{S}\) in \(\mathrm{SO}_{3}\) is similar to that of
2. \(\mathrm{p} \pi-\mathrm{d} \pi\) bond is present in \(\mathrm{SO}_{2}\)
4. \(\mathrm{SeF}_{4}\) and \(\mathrm{CH}_{4}\) have same shape
1) \(\mathrm{C}\) in \(\mathrm{C}_{2} \mathrm{H}_{2}\)
2) \(\mathrm{C}\) in \(\mathrm{C}_{2} \mathrm{H}_{4}\)
3) \(\mathrm{C}\) in \(\mathrm{CH}_{4}\)
4) \(\mathrm{C}\) in \(\mathrm{CO}_{2}\)
52. Allyl cyanide molecule contains
1) 9 sigma bonds, 4 pi bonds and no lone pair
2) 9 sigma bonds, 3 pi bonds and one lone pair
3) 8 sigma bonds, 5 pi bonds and one lone pair
4) 8 sigma bonds, 3 pi bonds and two lone pairs
53. All bond angles are exactly equal to \(109^{\circ} 28^{\text {! }}\) in :
1) methyl chloride 2) iodoform
3) chloroform
4) carbon tetrachloride
54. Which has the least bond angle
1) \(\mathrm{NH}_{3}\)
2) \(\mathrm{BeF}_{2}\)
3) \(\mathrm{H}_{2} \mathrm{O}\)
4) \(\mathrm{CH}_{4}\)
55. The shape of \(\mathrm{IF}_{6}^{-}\)is :
1) Trigonally distorted octahedron
2) Pyramidal
3) Octahedral
4) Square antiprism
56. Which of the following statements is not correct?
1) Double bond is shorter than a single bond
2) Sigma bond is weaker than a \(p\) (pi) bond
3) Double bond is stronger than a single bond
4) Covalent bond is stronger than hydrogen bond
57. In which of the following pair both the species have \(s p^{3}\) hybridization?
1) \(\mathrm{H}_{2} \mathrm{~S}, \mathrm{BF}_{3}\)
2) \(\mathrm{SiF}_{4}, \mathrm{BeH}_{2}\)
3) \(\mathrm{NF}_{3}, \mathrm{H}_{2} \mathrm{O}\)
4) \(\mathrm{NF}_{3}, \mathrm{BF}_{3}\)
58. Which of the following represents zero overlap of atomic orbitals.



(d) All of these
59. The structure of the noble gas compound \(\mathrm{XeF}_{4}\) is :
1) square planar
2) distorted tetrahedral
3) tetrahedral
4) octahedral
60. Which of the following pairs of species have identical shapes?
1) \(\mathrm{NO}_{2}^{+}\)and \(\mathrm{NO}_{2}^{-}\)
2) \(\mathrm{PCl}_{5}\) and \(\mathrm{BrF}_{5}\)
3) \(\mathrm{XeF}_{4}\) and \(\mathrm{ICI}_{4}^{-}\)
4) \(\mathrm{TeCl}_{4}\) and \(\mathrm{XeO}_{4}\)
61. Amongst \(\mathrm{NO}_{3}^{-}, \mathrm{AsO}_{3}^{3-}, \mathrm{CO}_{3}^{2-}, \mathrm{ClO}_{3}^{-}, \mathrm{SO}_{3}^{2-}\) and \(\mathrm{BO}_{3}^{3-}\), the non-planar species are
1) \(\mathrm{CO}_{3}^{2-}, \mathrm{SO}_{3}^{2-}, \mathrm{BO}_{3}^{3-}\)
2) \(\mathrm{AsO}_{3}^{3-}, \mathrm{ClO}_{3}^{-}, \mathrm{SO}_{3}^{2-}\)
3) \(\mathrm{NO}_{3}^{-}, \mathrm{CO}_{3}^{2-}, \mathrm{BO}_{3}^{3-}\)
4) \(\mathrm{SO}_{3}^{2-}, \mathrm{NO}_{3}^{-}, \mathrm{BO}_{3}^{3-}\)
62. What is the shape of the \(\mathrm{IBr}_{2}^{-}\)ion?
1) Linear
2) Bent shape with bond angle of about \(90^{\circ}\)
3) Bent shape with bond angle of about \(109^{\circ}\)
4) Bent shape with bond angle of about \(120^{\circ}\)
63. According to VSEPR theory, in which species do all the atoms lie in the same plane?
1. \(\mathrm{CH}_{3}^{+}\)
2. \(\mathrm{CH}_{3}^{-}\)
1) 1 only
2) 2 only
3) both 1 and 2
4) neither 1 nor 2
64. Which bonds are formed by a carbon atom with \(s p^{2}\)-hybridisation?
1) \(4 \pi\)-bonds
2) \(2 \pi\)-bonds and \(2 \sigma\)-bonds \(\quad\) 3) \(1 \pi\)-bonds and \(3 \sigma\)-bonds
4) \(4 \sigma\)-bonds
65. \(\mathrm{SF}_{2}, \mathrm{SF}_{4}\) and \(\mathrm{SF}_{6}\) have the hybridisation at sulphur atom respectively as :
1) \(s p^{2}, s p^{3}, s p^{2} d^{2}\)
2) \(s p^{3}, s p^{3}, s p^{3} d^{2}\)
3) \(s p^{3}, s p^{3} d, s p^{3} d^{2}\)
4) \(s p^{3}, s p d^{2}, d^{2} s p^{3}\)
66. The strength of bonds formed by \(s-s, p-p\) and \(s-p\) overlap is in the order of
1) \(s-p>s-s>p-p\)
2) \(p-p>s-s>s-p\)
3) \(s-s>p-p>s-p\)
4) \(s-s>s-p>p-p\)
\section{For more editable material contact \(@ 8977304976\)}
\section{TOPIC 4: MOT and Hydrogen Bonding}
67. The bond order in \(\mathrm{N}_{2}^{+}\)is
1) 1.5
2) 3.0
3) 2.5
4) 2.0
68. The molecular electronic configuration of \(\mathrm{H}_{2}^{+}\)ion is?
1) \((\sigma 1 \mathrm{~s})^{2}\)
2) \((\sigma 1 \mathrm{~s})^{2}\left(\sigma^{*} 1 s\right)^{2}\)
3) \((\sigma 1 s)^{2}\left(\sigma^{*} 1 s\right)^{1}\)
4) \((\sigma 1 \mathrm{~s})^{3}\)
69. In the change of \(\mathrm{NO}^{+}\)to \(\mathrm{NO}\), the electron is added to
1) \(\sigma\) - orbital
2) \(\pi\)-orbital
3) \(\sigma^{*}\) - orbital
4) \(\pi^{*}\) - orbital
70. The correct statement with regard to \(\mathrm{H}^{+}\)and \(\mathrm{H}^{-}\)is
1) Both \(\mathrm{H}^{+}\)and \(\mathrm{H}^{-}\)do not exist
2) \(\mathrm{H}_{2}^{-}\)is more stable than \(\mathrm{H}_{2}^{+}\)
3) \(\mathrm{H}_{2}^{+}\)is more stable than \(\mathrm{H}_{2}^{-}\)
4) Both \(\mathrm{H}_{2}^{+}\)and \(\mathrm{H}_{2}^{-}\)are equally stable
71. If \(\mathrm{N}_{\mathrm{x}}\) is the number of bonding orbitals of an atom and \(\mathrm{N}_{\mathrm{y}}\) is the number of antibonding orbitals, then the molecule/atom will be stable if
1) \(N_{x}>N_{y}\)
2) \(\mathrm{N}_{\mathrm{x}}=\mathrm{N}_{\mathrm{y}}\)
3) \(\mathrm{N}_{\mathrm{x}}<\mathrm{N}_{\mathrm{y}}\)
4) \(\mathrm{N}_{\mathrm{x}} \leq \mathrm{N}_{\mathrm{y}}\)
72. In an anti-bonding molecular orbital, electron density is minimum
1) around one atom of the molecule
2) between the two nuclei of the molecule
3) at the region away from the nuclei of the molecule
4) at no place
73. When two atomic orbitals combine, they form
1) one molecular orbital
2) two molecular orbital
3) three molecular orbital
4) four molecular orbital
74. Of the following hydrides which one has the lowest boiling point?
1) \(\mathrm{AsH}_{3}\)
2) \(\mathrm{SbH}_{3}\)
3) \(\mathrm{PH}_{3}\)
4) \(\mathrm{NH}_{3}\)
75. Which one of the following is the correct order of interactions?
1) covalent \(<\) hydrogen bonding \(<\) van der Waals \(<\) dipoledipole
2) van der Waals \(<\) hydrogen bonding \(<\) dipole-dipole \(<\) covalent
3) van der Waals \(<\) dipole-dipole \(<\) hydrogen bonding \(<\) covalent
4) dipole-dipole \(<\) van der Waals \(<\) hydrogen bonding \(<\) covalent
76. An ether is more volatile than an alcohol having the same molecular formula. This is due to
1) alcohols having resonance structures
2) intermolecular hydrogen bonding in ethers
3) intermolecular hydrogen bonding in alcohols
4) dipolar character of ethers
77. Paramagnetism is exhibited by molecules
1) not attracted into a magnetic field
2) containing only paired electrons
3) carrying a positive charge
4) containing unpaired electrons
78. Hydrogen bonding is maximum in :
1) \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\)
2) \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\)
3) \(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{C}=\mathrm{O}\)
4) \(\mathrm{CH}_{3} \mathrm{CHO}\)
79. What is the dominant intermolecular force or bond that must be overcome in converting liquid \(\mathrm{CH}_{3} \mathrm{OH}\) to a gas?
1) Dipole-dipole interaction
2) Covalent bonds
3) London dispersion force
4) Hydrogen bonding
80. In \(\mathrm{O}_{2}^{-}, \mathrm{O}_{2}\) and \(\mathrm{O}_{2}^{-2}\) molecular species, the total number of antibonding electrons respectively are
1) \(7,6,8\)
2) \(1,0,2\)
3) \(6,6,6\)
4) \(8,6,8\)

8. Theory: Redox Reaction


\section{Important Points to Remember}
Redox titration : Potassium permanganate is often used in redox titrations because it is a powerful oxidizing agent and serves as its own indicator. In acidic solutions, the purple \(\mathrm{MnO}_{4}^{-}\)ion is reduced to the nearly colourless \(\mathrm{Mn}^{2+}\) ion.
- The oxidation number of \(\mathrm{N}\) in \(\mathrm{NO}_{2}\) is +4 . In \(\mathrm{NO}\), the oxidation number is +2 .
- \(\mathrm{H}_{2} \mathrm{SO}_{4}\) is a non-oxidizing acid when cold and dilute.
- The strongest oxidizing agent in a solution of a "nonoxidizing" acid is \(\mathrm{H}^{+}\).
- When we balance the equation, the ion-electron method will tell us how \(\mathrm{H}^{+}\)and \(\mathrm{H}_{2} \mathrm{O}\) are involved in the reaction.
- Electrons must be added to whichever side of the halfreaction is more positive (or less negative).
- The charge on the polyatomic ion equals the sum of the oxidation numbers of its atoms.
- Mostly, the medium in which a redox reaction is to be balanced is given in the problem but if the problem does not state the medium explicitly, then the medium is decided by looking at the reactants or products. If an acid or base is one of the reactants or products, then the medium is the same.
- \(\mathrm{KMnO}_{4}\) acts as an oxidant in every medium although with different strength which follows the order acidic medium \(>\) neutral medium \(>\) alkaline medium
- Act as both oxidising and reducing agents : \(\mathrm{SO}_{2}, \mathrm{H}_{2} \mathrm{O}_{2}, \mathrm{O}_{3}, \mathrm{NO}_{2}\), etc.
- Nature of oxides based on oxidation number
(i) Lowest oxidation state \(\rightarrow\) Basic (MnO)
(ii) Intermediate oxidation state \(\rightarrow\) Amphoteric \(\left(\mathrm{Mn}_{3} \mathrm{O}_{4}, \mathrm{MnO}_{2}\right)\)
(iii) Highest oxidation state \(\rightarrow\) Acidic \(\left(\mathrm{Mn}_{2} \mathrm{O}_{7}\right)\)
D Ion-electron method-Acidic solution for Balancing the equation
Step 1 : Divide the equation into two half-reactions.
Step 2 : Balance atoms other than \(\mathrm{H}\) and \(\mathrm{O}\).
Step 3 : Balance \(\mathrm{O}\) by adding \(\mathrm{H}_{2} \mathrm{O}\).
Step 4 : Balance \(\mathrm{H}\) by adding \(\mathrm{H}^{+}\).
Step 5 : Balance net charge by adding \(\mathrm{e}^{-}\).
Step 6 : Make \(\mathrm{e}^{-}\)gain equal to \(\mathrm{e}^{-}\)loss, then add halfreactions.
Step 7 : Cancel anything that's the same on both sides.
Additional steps in the ion-electron method for basic
solutions :
Step 8 : Add to both sides of the equation the same number of \(\mathrm{OH}^{-}\)as there are \(\mathrm{H}^{+}\).
Step 9 : Combine \(\mathrm{H}^{+}\)and \(\mathrm{OH}^{-}\)to form \(\mathrm{H}_{2} \mathrm{O}\).
Step 10 : Cancel any \(\mathrm{H}_{2} \mathrm{O}\) that you can.
D Fractional Oxidation State:
Fractional oxidation state is the average oxidation state of the element. eg. \(\mathrm{S}_{4} \mathrm{O}_{6}^{2-}\) (tetrathionate ion), \(\mathrm{C}_{3} \mathrm{O}_{2}\) (carbon suboxide), and \(\mathrm{Br}_{3} \mathrm{O}_{8}\) (tribromooctaoxide)
D) Iodometric and Iodimetric titrations
\begin{tabular}{|c|l|c|}
\hline Estimation of & \multicolumn{1}{|c|}{ Reaction } & \(\begin{array}{c}\text { Reaction between oxidising } \\
\text { agent and reducing agent }\end{array}\)
\\\hline \(\mathrm{I}_{2}\) (Iodometry) & \(\begin{array}{l}\text { Titrating solution is } \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} .5 \mathrm{H}_{2} \mathrm{O}(\mathrm{Hypo}) \\
\mathrm{I}_{2}+2 \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} \rightarrow 2 \mathrm{NaI}_{2}+\mathrm{Na}_{2} \mathrm{~S}_{4} \mathrm{O}_{6} \\
\text { or } \mathrm{I}_{2}+2 \mathrm{~S}_{2} \mathrm{O}_{3}^{2-} \rightarrow 2 \mathrm{I}^{-}+\mathrm{S}_{4} \mathrm{O}_{6}^{2-}\end{array}\) & \(\begin{array}{l}\mathrm{I}_{2} \equiv 2 \mathrm{I}^{-} \equiv 2 \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} \\
w\left(\mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}\right)=\frac{M}{I}\end{array}\) \\
\hline \(\mathrm{CuSO}_{4}\) (Iodimetry) & \(\begin{array}{l}2 \mathrm{CuSO}_{4}+4 \mathrm{KI} \rightarrow \mathrm{Cu}_{2} \mathrm{I}_{2}+2 \mathrm{~K}_{2} \mathrm{SO}_{4}+\mathrm{I}_{2} \\
\text { or } 2 \mathrm{Cu}^{2+}+4 \mathrm{I}^{-} \rightarrow \mathrm{Cu}_{2} \mathrm{I}_{2}+\mathrm{I}_{2} \text { (white ppt.) }\end{array}\) & \(\begin{array}{l}2 \mathrm{CuSO}_{4} \equiv \mathrm{I}_{2} \equiv 2 \mathrm{I}^{-} \equiv 2 \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} \\
E w \text { of } \mathrm{CuSO}_{4}=\frac{M}{I}\end{array}\) \\
\hline
\end{tabular}
(i) \(\mathrm{S}_{4} \mathrm{O}_{6}^{2-}(4 x-12=-2, x=2.5)\)
Oxidation number of \(\mathrm{S}=2.5\)

(ii) \(\mathrm{C}_{3} \mathrm{O}_{2}(3 x-4=0, x=4 / 3)\)
Oxidation number of \(\mathrm{C}=4 / 3\)

(iii) \(\mathrm{Br}_{3} \mathrm{O}_{8}(3 x-16=0, x=16 / 3)\)
Oxidation number of \(\mathrm{Br}=16 / 3\)

9. EXERCISE-1 Redox Reaction
LEVEL-1
TOPIC 1: Oxidation and Reduction Reactions
1. Which quantities are conserved in all oxidation reduction reaction?
1) Charge only
2) Mass only
3) Both charges and mass
4) Neither charge nor mass

1) oxidised
2) reduced
3) precipitated
4) None of these
3. The conversion of sugar \(\mathrm{C}_{12} \mathrm{H}_{22} \mathrm{O}_{11} \rightarrow \mathrm{CO}_{2}\) is
1) oxidation
2) reduction
3) Both oxidation and reduction
4) Neither oxidation nor reduction
4. The product of oxidation of \(\mathrm{I}^{-}\)with \(\mathrm{MnO}_{4}^{-}\)in alkaline medium is
1) \(\mathrm{IO}^{-}\)
2) \(\mathrm{IO}_{3}^{-}\)
3) \(\mathrm{IO}_{4}^{-}\)
4) \(\mathrm{I}_{2}\)
5. In the following reaction, which is the species being oxidised ?
\(2 \mathrm{Fe}^{3+}(\mathrm{aq})+2 \mathrm{I}^{-}(\mathrm{aq}) \rightarrow \mathrm{I}_{2}(\mathrm{aq})+2 \mathrm{Fe}^{2+}(\mathrm{aq})\)
1) \(\mathrm{Fe}^{3+}\)
2) \(\mathrm{I}^{-}\)
3) \(\mathrm{I}_{2}\)
4) \(\mathrm{Fe}^{2+}\)
6. The compound that can work both as an oxidising as well as a reducing agent is
1) \(\mathrm{KMnO}_{4}\)
2) \(\mathrm{H}_{2} \mathrm{SO}_{4}\)
3) \(\mathrm{BaO}_{2}\)
4) \(\mathrm{H}_{2} \mathrm{O}_{2}\)
7. Which of the following substances acts as an oxidising as well as a reducing agent?
1) \(\mathrm{Na}_{2} \mathrm{O}\)
2) \(\mathrm{SnCl}_{2}\)
3) \(\mathrm{Na}_{2} \mathrm{O}_{2}\)
4) \(\mathrm{NaNO}_{2}\)
8. In the reaction \(2 \mathrm{FeCl}_{3}+\mathrm{H}_{2} \mathrm{~S} \rightarrow 2 \mathrm{FeCl}_{2}+2 \mathrm{HCl}+\mathrm{S}\)
1) \(\mathrm{FeCl}_{3}\) acts as an oxidising agent.
2) Both \(\mathrm{H}_{2} \mathrm{~S}\) and \(\mathrm{FeCl}_{3}\) are oxidised.
3) \(\mathrm{FeCl}_{3}\) is oxidised while \(\mathrm{H}_{2} \mathrm{~S}\) is reduced.
4) \(\mathrm{H}_{2} \mathrm{~S}\) acts as an oxidising agent.
9. When iron is rusted, it is
1) Oxidised
2) reduced
3) evaporated
4) decomposed
10. Which of the following is not an intermolecular redox reaction?
1) \(\mathrm{MgCO}_{3} \rightarrow \mathrm{MgO}+\mathrm{CO}_{2}\)
2) \(\mathrm{O}_{2}+2 \mathrm{H}_{2} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}\)
3) \(\mathrm{K}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{KOH}+(1 / 2) \mathrm{H}_{2}\)
4) \(\mathrm{MnBr}_{3} \rightarrow \mathrm{MnBr}_{2}+(1 / 2) \mathrm{Br}_{2}\)
11. In the reaction \(3 \mathrm{Mg}+\mathrm{N}_{2} \rightarrow \mathrm{Mg}_{3} \mathrm{~N}_{2}\)
1) magnesium is reduced
2) magnesium is oxidized
3) nitrogen is oxidized
4) None of these
12. One gas bleaches the colour of flowers by reduction, while the other by oxidation
1) \(\mathrm{CO}\) and \(\mathrm{Cl}_{2}\)
2) \(\mathrm{SO}_{2}\) and \(\mathrm{Cl}_{2}\)
3) \(\mathrm{H}_{2} \mathrm{~S}\) and \(\mathrm{Br}_{2}\)
4) \(\mathrm{NH}_{3}\) and \(\mathrm{SO}_{2}\)
13. In reaction of \(\mathrm{KMnO}_{4}\) and Mohr's salt, \(\mathrm{FeSO}_{4}\) is oxidised to
1) \(\mathrm{Fe}^{2+}\)
2) \(\mathrm{Fe}^{3+}\)
3) \(\mathrm{Fe}\)
4) All of these
14. Match the columns
Column-I
Column-II
1) \(2 \mathrm{Mg}+\mathrm{O}_{2} \rightarrow 2 \mathrm{MgO}\)
(p) Removal of hydrogen
2) \(\mathrm{Mg}+\mathrm{Cl}_{2} \rightarrow \mathrm{MgCl}_{2}\)
(q) Removal of electropositive element
3) \(2 \mathrm{H}_{2} \mathrm{~S}+\mathrm{O}_{2} \rightarrow 2 \mathrm{~S}+2 \mathrm{H}_{2} \mathrm{O}\)
(r) Addition of oxygen
4) \(2 \mathrm{KI}+\mathrm{H}_{2} \mathrm{O}+\mathrm{O}_{3} \rightarrow 2 \mathrm{KOH}+\mathrm{I}_{2}+\mathrm{O}_{2}\)
(s) Addition of electronegative element, chlorine
1) \(A-(s), B-(q), C-(p), D-(r)\)
2) \(A-(r), B-(s), C-(p), D-(q)\)
3) \(A-(s), B-(r), C-(q), D-(p)\)
4) \(A-(r), B-(p), C-(s), D-(q)\)
\section{TOPIC 2: Oxidation Number}
15. The oxidation number of chromium in potassium dichromate is
1) +6
2) -5
3) -2
4) +2
16. Phosphorus has the oxidation state of +3 in
1) phosphorous acid
2) orthophosphoric acid
3) hypophosphorous acid
4) metaphosphoric acid.
17. In which of the following reactions, nitrogen undergoes change in oxidation state?
1) \(\mathrm{NH}_{3}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{NH}_{4}^{+}+\mathrm{OH}^{-}\)
2) \(2 \mathrm{NO}_{2} \rightarrow \mathrm{N}_{2} \mathrm{O}_{4}\)
3) \(2 \mathrm{NO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{HNO}_{3}+\mathrm{HNO}_{2}\)
d) \(\mathrm{N}_{2} \mathrm{O}_{5}+\mathrm{H}_{2} \mathrm{O} \rightarrow 2 \mathrm{HNO}_{3}\)
18. The brown ring complex compound is formulated as \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5}(\mathrm{NO})\right] \mathrm{SO}_{4}\). The oxidation state of iron is
1) 1
2) 2
3) 3
4) 0
19. The pair of compounds in which both the metals are in the highest possible oxidation state is
1) \(\left[\mathrm{Fe}(\mathrm{CN})_{4}\right]^{4-},\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\)
2) \(\mathrm{CrO}_{2} \mathrm{Cl}_{2}, \mathrm{MnO}_{4}^{-}\)
3) \(\mathrm{TiO}_{3}, \mathrm{MnO}_{2}\)
4) \(\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}, \mathrm{MnO}_{3}\)
20. The oxidation number of \(\mathrm{S}\) in \(\mathrm{S}_{2} \mathrm{O}_{8}^{2-}\) is
1) +2
2) +4
3) +6
4) +7
21. The oxidation number of oxygen in \(\mathrm{O}_{2} \mathrm{PtF}_{6}\) is
1) -0.5
2) Zero
3) +0.5
4) +1
22. In which of the following compounds, iron has lowest oxidation state?
1) \(\mathrm{K}_{3}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\)
2) \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\)
3) \(\mathrm{FeSO}_{4} \cdot(\mathrm{NH} 4)_{2} \mathrm{SO}_{4} \cdot 6 \mathrm{H}_{2} \mathrm{O}\)
4) \(\mathrm{Fe}(\mathrm{CO})_{5}\)
23. In which of the compounds does 'manganese' exhibit highest oxidation number ?
1) \(\mathrm{MnO}_{2}\)
2) \(\mathrm{Mn}_{3} \mathrm{O}_{4}\)
3) \(\mathrm{K}_{2} \mathrm{MnO}_{4}\)
4) \(\mathrm{MnSO}_{4}\)
24. On reduction of \(\mathrm{KMnO}_{4}\) by oxalic acid in acidic medium, the oxidation number of Mn changes. What is the magnitude of this change?
1) From 7 to 2
2) From 6 to 2
3) From 5 to 2
4) From 7 to 4
25. When \(\mathrm{SO}_{2}\) is passed into an acidified potassium dichromate solution, the oxidation numbers of sulphur and chromium in the final products respectively are
1) \(+6,+6\)
2) \(+6,+3\)
3) \(0,+3\)
4) \(+2,+3\)
26. In which of the following coordination compounds does the transition metal have an oxidation number of +6 ?
a) \(\left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{Cl}\right] \mathrm{SO}_{4}\)
2) \(\mathrm{Cr}\left[\eta^{6}-\mathrm{C}_{6} \mathrm{H}_{6}\right]_{2}\)
c) \(\mathrm{K}_{2}\left[\mathrm{Cr}(\mathrm{CN})_{2} \mathrm{O}_{2}\left(\mathrm{O}_{2}\right) \mathrm{NH}_{3}\right]\)
d) \(\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{4}(\mathrm{SCN})_{2}\right]\left[\mathrm{CR}\left(\mathrm{NH}_{3}\right)_{2}(\mathrm{SCN})_{4}\right]\)
27. In which of the following transition metal complexes does the metal exhibit zero oxidation state ?
1) \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right] \mathrm{Cl}_{3}\)
2) \(\left.\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{SO}_{4} 3\right) \mathrm{Ni}(\mathrm{CO})_{4}\)
4) \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{X}_{3}\)
28. Oxidation numbers of chlorine atoms in \(\mathrm{CaOCl}_{2}\) are
1) 0,0
2) \(-1,-1\)
3) \(-1,+1\)
4) None of these
29. The oxide, which cannot act as a reducing agent, is
1) \(\mathrm{NO}_{2}\)
2) \(\mathrm{SO}_{2}\)
3) \(\mathrm{CO}_{2}\)
4) \(\mathrm{ClO}_{2}\)
30. Point out the correct statement of the following about \(\mathrm{Na}_{2} \mathrm{~S}_{4} \mathrm{O}_{6}\).
1) Average oxidation number of \(S\) atom is +2
2) Oxidation number of two \(S\) atoms is zero each and that of other two is +5 each
3) Oxidation number of two \(S\) atoms is +1 each and that of other two is +4 each
4) None of these
31. The oxidation state of the most electronegative element in the products of the reaction between \(\mathrm{BaO}_{2}\) and \(\mathrm{H}_{2} \mathrm{SO}_{4}\) are
1) 0 and -1
2) -1 and -2
3) -2 and 0
4) -2 and +1
32. When ethane is burnt in presence of excess of oxygen, the oxidation number of carbon changes by
1) +8
2) +7
3) +3
4) +4
\section{TOPIC 3: Disproportionation and Balancing of Redox Reactions}
33. Which of the following is not a redox reaction?
1) \(4 \mathrm{KClO}_{3} \rightarrow 3 \mathrm{KClO}_{4}+\mathrm{KCl}\)
2) \(\mathrm{Na}_{2} \mathrm{O}+2 \mathrm{HCl} \rightarrow 2 \mathrm{NaCl}+\mathrm{H}_{2} \mathrm{O}\)
3) \(\mathrm{SO}_{2}+2 \mathrm{H}_{2} \mathrm{~S} \rightarrow 2 \mathrm{H}_{2} \mathrm{O}+3 \mathrm{~S}\)
4) \(2 \mathrm{Na}+\mathrm{O}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{O}_{2}\)
34. For the reaction : \(\mathrm{NH}_{3}+\mathrm{OCl}^{-} \rightarrow \mathrm{N}_{2} \mathrm{H}_{4}+\mathrm{Cl}^{-}\)in basic medium, the coefficients of \(\mathrm{NH}_{3}, \mathrm{OCl}^{-}\)and \(\mathrm{N}_{2} \mathrm{H}_{4}\) for the balanced equation are respectively.
1) 2, 2, 2
2) 2, 2, 1
3) \(2,1,1\)
4) 4, 4, 2
35. In the reaction, \(\mathrm{Cl}_{2}+2 \mathrm{OH}^{-} \rightarrow \mathrm{OCl}^{-}+\mathrm{Cl}^{-}+\mathrm{H}_{2} \mathrm{O}\)
1) \(\mathrm{OH}^{-}\)is oxidising and \(\mathrm{Cl}_{2}\) is reducing agent 2) \(\mathrm{Cl}_{2}\) is oxidising and \(\mathrm{OH}^{-}\)is reducing agent
3) \(\mathrm{OH}^{-}\)is both oxidising and reducing agent 4) \(\mathrm{Cl}_{2}\) is both oxidising and reducing agent
36. When phosphorous reacts with caustic soda, the products are \(\mathrm{PH}_{3}\) and \(\mathrm{NaH}_{2} \mathrm{PO}_{2}\). This reaction is an example of
1) oxidation
2) reduction
3) disproportionation
4) none of these
37. \(\mathrm{KMnO}_{4}\) oxidises oxalic acid in acidic medium. The number of \(\mathrm{CO}_{2}\) molecules produced as per the balanced equation is
1) 10
2) 8
3) 6
4) 3
38. Which of the following does not represent redox reaction?
1) \(2 \mathrm{Ca}(\mathrm{OH})_{2}+\mathrm{Cl}_{2} \rightarrow \mathrm{Ca}(\mathrm{ClO})_{2}+\mathrm{CaCl}_{2}+2 \mathrm{H}_{2} \mathrm{O}\)
2) \(\mathrm{Cr}_{2} \mathrm{O}_{7}^{2-}+2 \mathrm{OH}^{-} \rightarrow 2 \mathrm{CrO}_{4}^{2-}+\mathrm{H}_{2} \mathrm{O}\)
3) \(\mathrm{NaIO}_{3}+\mathrm{NaHSO}_{3} \rightarrow \mathrm{NaHSO}_{4}+\mathrm{Na}_{2} \mathrm{SO}_{4}+\mathrm{I}_{2}+\mathrm{H}_{2} \mathrm{O}\) 4) \(2 \mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}+\mathrm{I}_{2} \rightarrow \mathrm{Na}_{2} \mathrm{~S}_{4} \mathrm{O}_{6}+2 \mathrm{NaI}\)
39. Amongst the following, the strongest oxidising agent is
1) \(\mathrm{KMnO}_{4}\)
2) \(\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}\)
3) \(\mathrm{H}_{2} \mathrm{O}_{2}\)
4) \(\mathrm{O}_{3}\)
40. Number of moles of \(\mathrm{K}_{2} \mathrm{Cr}_{2} \mathrm{O}_{7}\) reduced by one mole of \(\mathrm{Sn}^{2+}\) ions is
1) \(1 / 3\)
2) 3
3) \(1 / 6\)
4) 6
41. The reaction \(3 \mathrm{ClO}^{-}(\mathrm{aq}) \rightarrow \mathrm{ClO}_{3}^{-}(\mathrm{aq})+2 \mathrm{Cl}^{-}(\mathrm{aq})\) is an example of -
1) Oxidation reaction
2) Reduction reaction
3) Disproportionation reaction
4) Decomposition reaction
42. The species that undergoes disproportionation in an alkaline medium are
1) \(\mathrm{Cl}_{2}\)
2) \(\mathrm{MnO}_{4}^{2-}\)
3) \(\mathrm{NO}_{2}\)
4) All of these 43. What products are expected from the disproportionation reaction of hypochlorous acid?
1) \(\mathrm{HCl}\) and \(\mathrm{Cl}_{2} \mathrm{O}\)
2) \(\mathrm{HCl}\) and \(\mathrm{HClO}_{3}\)
3) \(\mathrm{HClO}_{3}\) and \(\mathrm{Cl}_{2} \mathrm{O}\)
4) \(\mathrm{HClO}_{2}\) and \(\mathrm{HClO}_{4}\)
44. Amongst the following which can act as an oxidising as well as reducing agent?
1) \(\mathrm{F}_{2}\)
2) \(\mathrm{SO}_{3}\)
3) \(\mathrm{H}_{2} \mathrm{O}_{2}\)
4) \(\mathrm{H}_{2} \mathrm{O}\)
45. The number of electrons lost in the following change is \(\mathrm{Fe}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{Fe}_{3} \mathrm{O}_{4}+\mathrm{H}_{2}\)
1) 2
2) 4
3) 6
4) 8
46. In which of the following reactions, \(\mathrm{SO}_{2}\) behaves as an oxidising agent?
1) \(2 \mathrm{MnO}_{4}^{-}+5 \mathrm{SO}_{2}+2 \mathrm{H}_{2} \mathrm{O} \rightarrow 5 \mathrm{SO}_{4}^{2-}+2 \mathrm{Mn}^{2+}+4 \mathrm{H}^{+}\)
2) \(\mathrm{Cl}_{2}+\mathrm{SO}_{2} \rightarrow \mathrm{SO}_{2} \mathrm{Cl}_{2}\)
3) \(2 \mathrm{H}_{2} \mathrm{~S}+\mathrm{SO}_{2} \rightarrow 3 \mathrm{~S}+2 \mathrm{H}_{2} \mathrm{O}\)
4) \(\mathrm{SO}_{2}+\mathrm{H}_{2} \mathrm{O} \rightarrow \mathrm{H}_{2} \mathrm{SO}_{3}\)
47. The oxidation numbers of \(\mathrm{C}-1, \mathrm{C}-2\) and \(\mathrm{C}-3\) in propyne \(\left(\stackrel{3}{\mathrm{C}} \mathrm{H}_{3} \stackrel{2}{\mathrm{C}} \equiv \stackrel{1}{\mathrm{C}} \mathrm{H}\right)\) respectively are
1) \(-1,0,-3\)
2) \(-1,1,-4\)
3) \(-2,0,-3\)
4) \(+1,-2,-3\)
\section{TOPIC 4: Electrode Potential}
48. Standard reduction potentials of the half reactions are given below :
\[
\begin{aligned}
& \mathrm{F}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{~F}^{-}(\mathrm{aq}) ; \mathrm{E}^{\circ}=+2.85 \mathrm{~V} \\
& \mathrm{Cl}_{2}(\mathrm{~g})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Cl}^{-} \text {(aq); } \mathrm{E}^{\circ}=+1.36 \mathrm{~V} \\
& \mathrm{Br}_{2}(\mathrm{l})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{Br}^{-}(\mathrm{aq}) ; \mathrm{E}^{\circ}=+1.06 \mathrm{~V} \\
& \mathrm{I}_{2}(\mathrm{~s})+2 \mathrm{e}^{-} \rightarrow 2 \mathrm{I}^{-} \text {(aq) } ; \mathrm{E}^{\circ}=+0.53 \mathrm{~V}
\end{aligned}
\]
The strongest oxidising and reducing agents respectively are :
1) \(\mathrm{F}_{2}\) and \(\mathrm{I}^{-}\)
2) \(\mathrm{Br}_{2}\) and \(\mathrm{Cl}^{-}\)
3) \(\mathrm{Cl}_{2}\) and \(\mathrm{Br}^{-}\)
4) \(\mathrm{Cl}_{2}\) and \(\mathrm{I}_{2}\)
49. Standard electrode potentials of redox couples
\(\mathrm{A}^{2+} / \mathrm{A}, \mathrm{B}^{2+} / \mathrm{B}, \mathrm{C} / \mathrm{C}^{2+}\) and \(\mathrm{D}^{2+} / \mathrm{D}\) are \(0.3 \mathrm{~V},-0.5 \mathrm{~V},-0.75 \mathrm{~V}\) and \(0.9 \mathrm{~V}\) respectively. Which of these is best oxidising agent and reducing agent respectively -
1) \(\mathrm{D}^{2+} / \mathrm{D}\) and \(\mathrm{B}^{2+} / \mathrm{B}\)
2) \(\mathrm{B}^{2+} / \mathrm{B}\) and \(\mathrm{D}^{2+} / \mathrm{D}\)
3) \(\mathrm{D}^{2+} / \mathrm{D}\) and \(\mathrm{C}^{2+} / \mathrm{C}\)
4) \(\mathrm{C}^{2+} / \mathrm{C}\) and \(\mathrm{D}^{2+} / \mathrm{D}\)
50. The standard electrode potentials of four elements A, B, C and D are \(-3.05,-1.66,-0.40\) and +0.80 . The highest chemical reactivity will be exhibited by :
1) \(A\)
2) B
3) \(C\)
4) \(\mathrm{D}\)

10. Group I & II


{Important Points to Remember}
- Atomic radii : \(\mathrm{Li}<\mathrm{Na}<\mathrm{K}<\mathrm{Rb}<\mathrm{Cs}\)
- Ionic radii : \(\mathrm{Li}^{+}<\mathrm{Na}^{+}<\mathrm{K}^{+}<\mathrm{Rb}^{+}<\mathrm{Cs}^{+}\)
- Electronegativity : \(\mathrm{Li}>\mathrm{Na}>\mathrm{K}>\mathrm{Rb}>\mathrm{Cs}\)
- First ionization potential : \(\mathrm{Li}>\mathrm{Na}>\mathrm{K}>\mathrm{Rb}>\mathrm{Cs}\)
- Melting point \(\mathrm{Li}>\mathrm{Na}>\mathrm{K}>\mathrm{Rb}>\mathrm{Cs}\)
- Colour of the flame : Li - Red, Na - Golden, K - Violet, Rb - Red, Cs - Blue, Ca - Brick red, Sr - Blood red, Ba-Apple green \(4 \mathrm{Rb}\) and \(\mathrm{Cs}\) show photoelectric effect.
- Stability of hydrides : \(\mathrm{LiH}>\mathrm{NaH}>\mathrm{KH}>\mathrm{RbH}>\mathrm{CsH}\)
- The finely divided BaSO4 is called blanc fire and used in paints.
- Pure \(\mathrm{Ca}\left(\mathrm{H}_{2} \mathrm{PO}_{4}\right)_{2}\) is used as american baking powder.
- Melting point:
For the same alkali metal, the melting points decrease in the order:
Fluoride \(>\) chloride \(>\) bromide \(>\) iodide
This is due to the decrease in lattice enthalpies as the size of the halide ion increases (size of cation is constant).
v \(\quad\) The stability of peroxides and superoxides increases in the order
\(\mathrm{O}_{2}<\mathrm{K}_{2} \mathrm{O}_{2}<\mathrm{Rb}_{2} \mathrm{O}_{2}<\mathrm{Cs}_{2} \mathrm{O}_{2} \mathrm{NaO}_{2}<\mathrm{KO}_{2}<\mathrm{RbO}_{2}<\mathrm{CsO}_{2}\)
B) Basic strength of oxides and hydroxides
\(\mathrm{BeO}<\mathrm{MgO}<\mathrm{CaO}<\mathrm{SrO}<\mathrm{BaO}\)
\(\mathrm{Be}(\mathrm{OH})_{2}<\mathrm{Mg}(\mathrm{OH})_{2}<\mathrm{Ca}(\mathrm{OH})_{2}<\mathrm{Sr}(\mathrm{OH})_{2}<\mathrm{Ba}(\mathrm{OH})_{2}\)
D Solubility of hydroxides
\(\mathrm{Be}(\mathrm{OH})_{2}<\mathrm{Mg}(\mathrm{OH})_{2}<\mathrm{Ca}(\mathrm{OH})_{2}<\mathrm{Sr}(\mathrm{OH})_{2}<\mathrm{Ba}(\mathrm{OH})_{2}\)
D Solubility of sulphates
\(\mathrm{BeSO}_{4}>\mathrm{MgSO}_{4}>\mathrm{CaSO}_{4}>\mathrm{SrSO}_{4}>\mathrm{BaSO}_{4}\)
H Solubility of carbonates
\(\mathrm{BeCO}_{3}>\mathrm{MgCO}_{3}>\mathrm{CaCO}_{3}>\mathrm{SrCO}_{3}>\mathrm{BaCO}_{3}\)
» \(\mathrm{CuBr}\) is more covalent than \(\mathrm{NaBr}\), although \(\mathrm{Cu}^{+}\)and \(\mathrm{Na}^{+}\)have the same charge +1 , and nearly the same size, i.e., \(\mathrm{Cu}^{+}(0.96 \stackrel{0}{\mathrm{~A}})\) and \(\mathrm{Na}+(1.02 \stackrel{0}{\mathrm{~A}})\). This is due to the fact that \(\mathrm{Cu}^{+}\)ion has a pseudo noble gas configuration, i.e., having \(18 e^{-9} \mathrm{~s}\) in the outermost shell.
D Peroxide \(\left(\mathrm{O}_{2}^{2-}\right)\) of the first group are colourless and diamagnetic while superoxide \(\left(\mathrm{O}_{2}^{-}\right)\)are coloured and paramagnetic.
H Except \(\mathrm{Cs}_{2} \mathrm{O}\), all other monoxide, e.g., \(\mathrm{Li}_{2} \mathrm{O}, \mathrm{Na}_{2} \mathrm{O}, \mathrm{K}_{2} \mathrm{O}\) and \(\mathrm{Rb}_{2} \mathrm{O}\) have antiflourite structures, \(\mathrm{Cs}_{2} \mathrm{O}\) has anti \(\mathrm{CdCl}_{2}\) layer structure.
\(\mathrm{KO}_{2}\) and \(\mathrm{Na}_{2} \mathrm{O}_{2}\) are used as a source of oxygen (or for the purification of air) in confined space such as submarines, space shuttles and in emergency breathing instrument such as oxygen masks.
11. Exercise Group I & II
TOPIC 1: Preparation and Properties of Alkali Metals and their Compounds}
1. Which of the following alkali metal is highly radioactive?
1) Rubidium
2) Caesium
3) Francium
4) Both 1) and 3)
2. Which compound will show the highest lattice energy ?
1) \(\mathrm{RbF}\)
2) \(\mathrm{CsF}\)
3) \(\mathrm{NaF}\)
4) \(\mathrm{KF}\)
3. Strongest bond is in between
1) \(\mathrm{CsF}\)
2) \(\mathrm{NaCl}\)
3) \(\mathrm{LiBr}\)
4) None of above 4. In crystals which one of the following ionic compounds would you expect maximum distance between centres of cations and anions?
1) \(\mathrm{LiF}\)
2) CsF
3) CsI
4) LiI
5. Which one of the alkali metals, forms only, the normal oxide, \(\mathrm{M}_{2} \mathrm{O}\) on heating in air ?
1) \(\mathrm{Rb}\)
2) \(\mathrm{K}\)
3) \(\mathrm{Li}\)
4) \(\mathrm{Na}\)
6. The ionic mobility of alkali metal ions in aqueous solution is maximum for
1) \(\mathrm{Li}^{+}\)
2) \(\mathrm{Na}^{+}\)
3) \(\mathrm{K}^{+}\)
4) \(\mathrm{Rb}^{+}\)
7. Which of the following pairs of substances would give same gaseous product on reaction with water?
1) \(\mathrm{Na}\) and \(\mathrm{Na}_{2} \mathrm{O}_{2}\)
2) \(\mathrm{Ca}\) and \(\mathrm{CaH}_{2}\)
3) \(\mathrm{Ca}\) and \(\mathrm{CaO}\)
4) \(\mathrm{Ba}\) and \(\mathrm{BaO}_{2}\)
8. Which of the following statements is incorrect?
1) Alkali metal hydroxide are hygroscopic.
2) Dissolution of Alkali metal hydroxide is endothermic.
3) Aqueous solution of alkali metal hydroxides are strongly basic.
4) Alkali metal hydroxides form ionic crystals.
9. Which is most basic in character?
1) \(\mathrm{CsOH}\)
2) \(\mathrm{KOH}\)
3) \(\mathrm{NaOH}\)
4) \(\mathrm{LiOH}\)
10. An inorganic compound which on heating first melts, then solidifies and liberates \(\mathrm{O}_{2}\) gas, the inorganic compound is
1) \(\mathrm{Al}_{2} \mathrm{O}_{3}\)
2) \(\mathrm{KMnO}_{4}\)
3) \(\mathrm{MnO}_{2}\)
4) \(\mathrm{KClO}_{3}\)
11. The element which on burning in air gives peroxide is
1) lithium
2) sodium
3) rubidium
4) cesium
12. Which of the following is known as fusion mixture ?
1) Mixture of \(\mathrm{Na}_{2} \mathrm{CO}_{3}+\mathrm{NaHCO}_{3}\)
2) \(\mathrm{Na}_{2} \mathrm{CO}_{3} \cdot 10 \mathrm{H}_{2} \mathrm{O}\)
3) Mixture of \(\mathrm{K}_{2} \mathrm{CO}_{3}+\mathrm{Na}_{2} \mathrm{CO}_{3}\)
4) \(\mathrm{NaHCO}_{3}\)
13. Which of the following has density greater than water?
1) \(\mathrm{Li}\)
2) \(\mathrm{Na}\)
3) \(\mathrm{K}\)
4) \(\mathrm{Rb}\)
14. Among LiI, NaI, KI, the one which is more ionic and more soluble in water is :
1) \(\mathrm{KI}\)
2) \(\mathrm{NaI}\)
3) LiI
4) None of these
15. Which of the following metal is used along with lithium to make the alloy named 'white metal'?
1) Nickel
2) Aluminium
3) Silver
4) Lead
16. The correct order of radii is -
1) \(\mathrm{Li}<\mathrm{Be}<\mathrm{Mg}\)
2) \(\mathrm{H}^{+}<\mathrm{Li}^{+}<\mathrm{H}^{-}\)
3) \(\mathrm{Mn}^{3+}<\mathrm{Mn}^{2+}<\mathrm{Mn}^{7+}\)
4) \(\mathrm{K}^{+}>\mathrm{Cl}^{-}>\mathrm{S}^{2-}\)
17. The melting point of lithium \(\left(181^{\circ} \mathrm{C}\right)\) is just double the melting point of sodium \(\left(98{ }^{\circ} \mathrm{C}\right)\) because -
1) down the group, the hydration energy decreases.
2) down the group, the ionization energy decreases.
3) down the group the cohesive energy decreases.
4) none of these.
18. Suppose an element is kept in air chamber, than air content was evaluated after sometime, oxygen and nitrogen content was found to be low comparitively. The given element will be
1) \(\mathrm{Li}\)
2) \(\mathrm{Rb}\)
3) \(\mathrm{Na}\)
4) \(\mathrm{K}\)
19. Why lithium react less vigorously with water than other alkali metals?
1) Lithium has most negative E- value
2) Lithium has small size and very high hydration energy.
3) Lithium has least negative E- value
4) Both 1) and 2)
20. Fires, that result from the combustion of alkali metals can be extinguished by
1) \(\mathrm{CCl}_{4}\)
2) sand
3) water
4) kerosene
21. In the replacement reaction
\(\frac{\lambda}{\nearrow} \mathrm{CI}+\mathrm{MF} \longrightarrow \frac{\lambda}{\nearrow} \mathrm{CF}+\mathrm{MI}\)
The reaction will be most favourable if \(M\) happens to be :
1) \(\mathrm{Na}\)
2) \(\mathrm{K}\)
3) \(\mathrm{Rb}\)
4) \(\mathrm{Li}\)
22. Magnitude of which of the following property of alkali metals increases with the increase of atomic number
1) Electronegativity
2) Ionic radius 3) First ionization energy
4) Melting point
23. Alkali metals form peroxides and superoxides except
1) \(\mathrm{Na}\)
2) \(\mathrm{Rb}\)
3) \(\mathrm{Li}\)
4) \(\mathrm{Cs}\)
24. Which of the following is used as a source of oxygen in space capsules, submarines and breathing masks ?
1) \(\mathrm{Li}_{2} \mathrm{O}\)
2) \(\mathrm{Na}_{2} \mathrm{O}_{2}\)
3) \(\mathrm{KO}_{2}\)
4) \(\mathrm{K}_{2} \mathrm{O}_{2}\)
25. Which is an ore of potassium?
1) Carnellite
2) Cryolite
3) Bauxite
4) Dolomite
26. Which of the following statements is correct for CsBr3?
1) It is a covalent compound.
2) It contains \(\mathrm{Cs}^{3+}\) and \(\mathrm{Br}^{-}\)ions.
3) It contains \(\mathrm{Cs}^{+}\)and \(\mathrm{Br}_{3}^{-}\)ions
4) It contains \(\mathrm{Cs}^{+}\), and \(\mathrm{Br}^{-}\)and lattice \(\mathrm{Br}_{2}\) molecule.
27. When potassium dichromate crystal are heated with conc. \(\mathrm{HCl}\)
1) \(\mathrm{O}_{2}\) is evolved
2) Chromyl chloride vapours are evolved
3) \(\mathrm{Cl}_{2}\) is evolved
4) No reaction takes place
28. An alloy of which pair of metals is liquid at ordinary temperature and is used in special thermometers used for finding temperatures above the boiling point of mercury.
1) \(\mathrm{Na}\) and \(\mathrm{Hg}\)
2) \(\mathrm{K}\) and \(\mathrm{Hg}\)
3) \(\mathrm{Na}\) and \(\mathrm{K}\)
4) None of these
29. An alkaline metal oxide which is soluble in \(\mathrm{NaOH}\) has
1) \(\mathrm{NaCl}\) structure
2) wurzite structure
3) CsCl structure
4) rutile structure
30. The alkali metals which form normal oxide, peroxide as well as a super oxides are
1) \(\mathrm{Na}, \mathrm{Li}\)
2) \(\mathrm{K}, \mathrm{Li}\)
3) Li, Cs
4) \(\mathrm{K}, \mathrm{Rb}\)
31. Which of the following compounds are paramagnetic in nature?
1) \(\mathrm{KO}_{2}\)
2) \(\mathrm{K}_{2} \mathrm{O}_{2}\)
3) \(\mathrm{Na}_{2} \mathrm{O}_{2}\)
4) \(\mathrm{Rb}_{2} \mathrm{O}_{2}\)
\section{TOPIC 2: Some Important Compounds of Sodium}
32. When sulphur is heated with \(\mathrm{NaOH}(\mathrm{aq})\) ? The compounds formed are
1) \(\mathrm{Na}_{2} \mathrm{~S}+\mathrm{H}_{2} \mathrm{O}\)
2) \(\mathrm{Na}_{2} \mathrm{SO}_{3}+\mathrm{H}_{2} \mathrm{O}\)
3) \(\mathrm{Na}_{2} \mathrm{~S}+\mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}\)
4) \(\mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3}+\mathrm{H}_{2} \mathrm{O}\)
33. Sodium carbonate is manufactured by Solvay process. The products which can be recycled are
1) \(\mathrm{CO}_{2}\) and \(\mathrm{NH}_{3}\)
2) \(\mathrm{CO}_{2}\) and \(\mathrm{NH}_{4} \mathrm{Cl}\)
3) \(\mathrm{NaCl}\) and \(\mathrm{CaO}\)
4) \(\mathrm{CaCl}_{2}\) and \(\mathrm{CaO}\).
34. An aqueous solution of salt ' \(\mathrm{R}\) ' when treated with dil. \(\mathrm{HCl}\), a colourless gas is given out. The gas so evolved when passed through acidified \(\mathrm{KMnO}_{4}\) decolourises \(\mathrm{KMnO}_{4}\) solution. The salt ' \(\mathrm{R}\) ' is
1) \(\mathrm{Na}_{2} \mathrm{CO}_{3}\)
2) \(\mathrm{NaClO}_{3}\)
3) \(\mathrm{NaNO}_{2}\)
4) \(\mathrm{Na}_{2} \mathrm{SO}_{3}\)
35. How \(\mathrm{NH}_{3}\) is recovered in Solvay process?
1) By reaction of \(\mathrm{NH}_{4} \mathrm{Cl}\) and \(\mathrm{Ca}(\mathrm{OH})_{2}\)
2) By reaction of \(\mathrm{NH}_{4} \mathrm{HCO}_{3}\) and \(\mathrm{NaCl}\)
3) By reaction of \(\left(\mathrm{NH}_{4}\right)_{2} \mathrm{CO}_{3}\) with \(\mathrm{H}_{2} \mathrm{O}\)
4) By any of the above
36. Baking powder contains :
1) \(\mathrm{NaHCO}_{3}, \mathrm{Ca}\left(\mathrm{H}_{2} \mathrm{PO}_{2}\right)_{2}\) and starch
2) \(\mathrm{NaHCO}_{3}, \mathrm{Ca}\left(\mathrm{H}_{2} \mathrm{PO}_{2}\right)_{2}\)
3) \(\mathrm{NaHCO}_{3}\), starch
4) \(\mathrm{NaHCO}_{3}\)
37. Sodium thiosulphate, \(\mathrm{Na}_{2} \mathrm{~S}_{2} \mathrm{O}_{3} .5 \mathrm{H}_{2} \mathrm{O}\) is used in photography to
1) reduce the silver bromide grains to metallic silver.
2) convert the metallic silver to silver salt.
3) remove undecomposed \(\mathrm{AgBr}\) as soluble silver thiosulphate complex. 4) remove reduced silver.
38. Sodium carbonate solution in water is alkaline due to
1) hydrolysis of \(\mathrm{Na}^{+}\)
2) hydrolysis of \(\mathrm{CO}_{3}^{2-}\)
3) hydrolysis of both \(\mathrm{Na}^{+}\)and \(\mathrm{CO}_{3}^{2-}\) ions
4) none of these
39. Which of the following statements is incorrect?
1) Pure sodium metal dissolves in liquid ammonia to give blue solution.
2) \(\mathrm{NaOH}\) reacts with glass to give sodium silicate.
3) Aluminium reacts with excess \(\mathrm{NaOH}\) to give \(\mathrm{Al}(\mathrm{OH})_{3}\)
4) \(\mathrm{NaHCO}_{3}\) on heating gives \(\mathrm{Na}_{2} \mathrm{CO}_{3}\)
40. Fire extinguishers contain \(\mathrm{H}_{2} \mathrm{SO}_{4}\) and which one of the following?
1) \(\mathrm{NaHCO}_{3}\) and \(\mathrm{Na}_{2} \mathrm{CO}_{3}\)
2) \(\mathrm{Na}_{2} \mathrm{CO}_{3}\)
3) \(\mathrm{NaHCO}_{3}\)
4) \(\mathrm{CaCO}_{3}\)
41. In Castner-Kellner cell for production of sodium hydroxide:
1) Brine is electrolyzed with Pt electrodes 2) Brine is electrolyzed using graphite electrodes 3) Molten sodium chloride is electrolysed 4) Sodium amalgam is formed at mercury cathode 42. Sodium cobaltinitrite is used in the detection of
1) \(\mathrm{K}\)
2) \(\mathrm{Ca}\)
3) \(\mathrm{Sr}\)
4) \(\mathrm{Ba}\)
TOPIC 3: Preparation and Properties of Alkaline Earth Metals and their Compounds
43. Of the metals Be, Mg, Ca and Sr of group II A. In the periodic table the least ionic chloride would be formed by
1) \(\mathrm{Be}\)
2) \(\mathrm{Mg}\)
3) \(\mathrm{Ca}\)
4) \(\mathrm{Sr}\)
44. Which one is known as barytes?
1) \(\mathrm{BaSO}_{4}\)
2) \(\mathrm{BaCl}_{2} \cdot \mathrm{HH}_{2} \mathrm{O}\)
3) \(\mathrm{BaO}\)
4) \(\mathrm{BaCO}_{3}\)
45. Which of the following will precipitate first when aqueous solution containing sulphate ions are added?
1) \(\mathrm{Mg}^{2+}\)
2) \(\mathrm{Ca}^{2+}\)
3) \(\mathrm{Sr}^{2+}\)
4) \(\mathrm{Ba}^{2+}\)
46. Which of the following compounds is used in preparation of green fire ?
1) \(\mathrm{K}_{2} \mathrm{SO}_{4}\)
2) \(\mathrm{NaNO}_{3}\)
3) \(\mathrm{Ba}\left(\mathrm{NO}_{3}\right)_{2}\)
4) None of these
47. Lithopone is
1) \(\mathrm{BaO}+\mathrm{ZnSO}_{4}\)
2) \(\mathrm{BaS}+\mathrm{ZnSO}_{4}\)
3) \(\mathrm{ZnS}+\mathrm{BaSO}_{4}\)
4) \(\mathrm{ZnO}+\mathrm{BaSO}_{4}\)
48. Mixture of \(\mathrm{MgCl}_{2}\) and \(\mathrm{MgO}\) is called :
1) portland cement
2) sorel's cement
3) double salt
4) none of these
49. Which of the following statement(s) is/are correct regarding \(\mathrm{Al}\) and \(\mathrm{Be}\) ?
(i) Both of these react with alkali.
(ii) There is diagonal relationship among these elements.
1) Both (i) and (ii)
2) Only (i)
3) Only (ii)
4) Neither (i) nor (ii)
50. Philosopher's wool on heating with \(\mathrm{BaO}\) at \(1100^{\circ} \mathrm{C}\) produces:
1) \(\mathrm{Ba}+\mathrm{ZnCl}_{2}\)
2) \(\mathrm{BaCdO}_{2}\)
3) \(\mathrm{BaZnO}_{2}\)
4) \(\mathrm{BaO}_{2}+\mathrm{Zn}\)
51. Arrange the following compounds in order of increasing solubility
(i) \(\mathrm{MgF}_{2}\)
(ii) \(\mathrm{CaF}_{2}\)
(iii) \(\mathrm{BaF}_{2}\)
1) (i) < (ii) < (iii)
2) (ii) < (i) < (iii)
3) (ii) < (iii) < (ii)
4) (iii) < (ii) < (i)
52. The first ionization potential of \(\mathrm{Mg}\) is
1) less than \(\mathrm{Al}\)
2) more than \(\mathrm{Al}\)
3) equal to \(\mathrm{Al}\)
4) can be less or more than \(\mathrm{Al}\)
53. Calcium is obtained by the
1) electrolysis of solution of calcium chloride in water
2) electrolysis of molten anhydrous calcium chloride or fused calcium chloride
3) roasting of limestone
4) reduction of calcium chloride with carbon
54. \(\mathrm{BaO}_{2}\) and ozone reacts to produce
1) \(\mathrm{Ba}\)
2) \(\mathrm{Ba}_{2} \mathrm{O}_{3}\)
3) \(\mathrm{BaO}\)
4) \(\mathrm{Ba}(\mathrm{OH})_{3}\)
\section{TOPIC 4: Some Important Compounds of Calcium}
55. Which one of the following is flourspar?
1) \(\mathrm{CaF}_{2}\)
2) \(\mathrm{CaO}\)
3) \(\mathrm{H}_{2} \mathrm{~F}_{2}\)
4) \(\mathrm{CaCO}_{3}\)
56. Gypsum on heating at \(120-130^{\circ} \mathrm{C}\) gives :
1) anhydrous salt
2) hemihydrate
3) monohydrate
4) dihydrate
57. Melting point of calcium halides decreases in the order
1) \(\mathrm{CaF}_{2}>\mathrm{CaCl}_{2}>\mathrm{CaBr}_{2}>\mathrm{CaI}_{2}\)
2) \(\mathrm{CaI}_{2}>\mathrm{CaBr}_{2}>\mathrm{CaCl}_{2}>\mathrm{CaF}_{2}\)
3) \(\mathrm{CaBr}_{2}>\mathrm{CaI}_{2}>\mathrm{CaF}_{2}>\mathrm{CaCl}_{2}\)
4) \(\mathrm{CaCl}_{2}>\mathrm{CaBr}_{2}>\mathrm{CaI}_{2}>\mathrm{CaF}_{2}\)
58. The substance not likely to contain \(\mathrm{CaCO} 3\) is
1) gypsum
2) sea shells
3) dolomite
4) a marble statue
59. Bone ash contains
1) \(\mathrm{CaO}\)
2) \(\mathrm{CaSO}_{4}\)
3) \(\mathrm{Ca}_{3}\left(\mathrm{PO}_{4}\right)_{2}\)
4) \(\mathrm{Ca}\left(\mathrm{H}_{2} \mathrm{PO}_{4}\right)_{2}\)
60. Oxygen is obtained from bleaching powder by
1) the action of dilute acid
4) the action of alkali
3) heating it with lime
4) heating it with cobalt salt

12. Theory States of matter


Important Points to Remember}
(i) \(C_{p}-C_{v}=\mathrm{R}=2 \mathrm{cal}=8.314 \mathrm{~J}\)
(ii) \(\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=\frac{3}{2} \mathrm{R}\)
(iii) For monoatomic gas, \(C_{v}=3\) calories
(iv) For monoatomic gases, \(\frac{C_{p}}{C_{v}}=\frac{3}{5}=1.66\)
(v) For diatomic gases, \(=\frac{\mathrm{C}_{\mathrm{p}}}{\mathrm{C}_{\mathrm{v}}}=1.40\)
\(\left(\mathrm{C}_{\mathrm{v}}=\frac{5}{3} \mathrm{R}, \mathrm{C}_{\mathrm{p}}=\frac{7}{2} \mathrm{R}\right)\)
(vi) For Triatomic gases \(\frac{C_{p}}{C_{v}}=1.33\)
- Viscosity
(i) Effect of temperature on viscosity: Viscosity decreases with increase in temperature. The relationship between coefficient of viscosity, \(\eta\) and absolute temperature \(\mathrm{T}\) is \(\eta=A \mathrm{e}^{\mathrm{Ea} / \mathrm{RT}}\) where \(A\) and \(E_{a}\) are constants for a given liquid
(ii) Effect of pressure on viscosity: Viscosity increases with increase in pressure.
- Collision frequency : The total number of collisions occurring in a unit volume of a gas per second under given set of conditions.
\(\mathrm{z} \propto \mathrm{T}^{2 / 3}\) (at constant
\(\mathrm{Z} \propto \mathrm{P}^{2}\) (at constant \(\mathrm{T}\) )
- Joule-Thomson effect : When a compressed gas is allowed to expand through a small orifice, cooling effect is caused and temperature falls. This is known as Joule Thomson effect which is observed only if the gas below certain temperature called inversion temperature.
- Greater the value of ' \(a\) ' more easily the gas is liquefiable \(a=\frac{{ }^{2}}{n^{2}}\)
\[
a=\frac{P V^{2}}{n^{2}}
\]
- Density and molar mass of a gas: According to the ideal gas equation \(P V=n R T\),
\[
\frac{\mathrm{P}_{1} \mathrm{~V}_{1}}{\mathrm{~T}_{1}}=\frac{\mathrm{P}_{2} \mathrm{~V}_{2}}{\mathrm{~T}_{2}}
\]
In terms of density, we get
\[
\mathrm{P}=\frac{\mathrm{m}}{\mathrm{M}_{\mathrm{w}}} \frac{\mathrm{RT}}{\mathrm{V}}=\frac{\mathrm{m}}{\mathrm{V}} \frac{\mathrm{RT}}{\mathrm{M}_{\mathrm{w}}}=\mathrm{d} \frac{\mathrm{RT}}{\mathrm{M}_{\mathrm{w}}}
\]
- STP (Standard temperature and pressure) or NTP (normal temperature and pressure) conditions are \(\mathrm{T}=\) \(0^{\circ} \mathrm{C}\)
\(=273.15 \mathrm{~K}, \mathrm{P}=1 \mathrm{~atm}\) or \(\mathrm{T}=0{ }^{\circ} \mathrm{C}=273.15 \mathrm{~K}, \mathrm{P}=1 \mathrm{bar}\)
(ii) Volume of a gas at STP \((\mathrm{P}=1 \mathrm{bar})=22.71098 \mathrm{~L} \mathrm{~mol}_{-1}=22.7 \mathrm{~L} \mathrm{~mol}_{-1}\)
\(1 \mathrm{~atm}=76 \mathrm{~cm}=760 \mathrm{~mm}=760\) torr
\(=101325 \mathrm{~Pa}\) or \(\mathrm{N} \mathrm{m}_{-2}=1.01325^{-} 1_{-5} \mathrm{~Pa}\) or \(\mathrm{N} \mathrm{m}_{-2}=10_{5} \mathrm{~Pa}\) or \(\mathrm{N} \mathrm{m}-2\)
\(1 \mathrm{~atm}=1.013 \mathrm{bar}\)
\(1 \mathrm{bar}=0.987 \mathrm{~atm}=10_{2} \mathrm{~K} \mathrm{~Pa}\)
\(1 \mathrm{~atm}=0.06805 \mathrm{psi}\)
\(1 \mathrm{Nm}_{-2}=6894.8 \mathrm{psi}\)
D According to Graham's Law of diffusion, under similar conditions of temperature and pressure, if \(r_{1}\) and \(r_{2}\) are rates of diffusion of two gases with densities \(d_{1}\) and \(d_{2}\) then
\[
\frac{\mathrm{r}_{1}}{\mathrm{r}_{2}}=\sqrt{\frac{\mathrm{d}_{2}}{\mathrm{~d}_{1}}}=\sqrt{\frac{\mathrm{M}_{\mathrm{w} 2}}{\mathrm{M}_{\mathrm{w} 1}}}
\]
For gases at different pressures, \(\left(\mathrm{r} \propto \frac{\mathrm{P}}{\sqrt{\mathrm{M}_{\mathrm{w}}}}\right) \frac{\mathrm{r}_{1}}{\mathrm{r}_{2}}=\frac{\mathrm{P}_{1}}{\mathrm{P}_{2}} \sqrt{\frac{\mathrm{M}_{\mathrm{w}_{1}}}{\mathrm{M}_{\mathrm{w}_{2}}}}\)
\(\triangleright\) For gases at different temperatures, \(\left(\mathrm{r} \propto \sqrt{\frac{\mathrm{T}}{\mathrm{M}_{\mathrm{w}}}}\right) \frac{\mathrm{r}_{1}}{\mathrm{r}_{2}}=\sqrt{\frac{\mathrm{T}_{1} \mathrm{M}_{\mathrm{w}_{1}}}{\mathrm{~T}_{2} \mathrm{M}_{\mathrm{w}_{1}}}}\)
Most probable velocity, \(\alpha=\sqrt{\frac{2 \mathrm{RT}}{\mathrm{M}_{\mathrm{w}}}}\)
A) Average velocity, \(\mathrm{n}=\mathrm{v}=\sqrt{\frac{8 \mathrm{RT}}{\pi \mathrm{M}_{\mathrm{w}}}}\)
\(\Delta \mathrm{RMS}\) velocity, \(\mathrm{u}=\sqrt{\frac{3 \mathrm{RT}}{\mathrm{M}_{\mathrm{w}}}}=\sqrt{\frac{3 \mathrm{RT}}{\mathrm{M}_{\mathrm{w}}}}=\sqrt{\frac{3 \mathrm{P}}{\mathrm{d}}}\)
- A gas can be liquefied by cooling or by applying pressure or by the combined effect of both. However, the effect of temperature is more important because for every gas there is a particular temperature above which it cannot be liquefied howsoever high pressure is applied.
Critical temperature \(T_{c}=\frac{8 a}{27 R b}\)
D Critical pressure: \(\quad \mathrm{P}_{\mathrm{c}}=\frac{\mathrm{a}}{27 \mathrm{~b}^{2}}\)
D Critical volume \(\left(V_{c}\right) \quad \mathrm{V}_{\mathrm{c}}=3 \mathrm{~b}\)
D) Critical compressibility factor: \(\quad \mathrm{Z}_{\mathrm{c}}=\frac{\mathrm{P}_{\mathrm{c}} \mathrm{V}_{\mathrm{c}}}{\mathrm{RT}_{\mathrm{c}}}=\frac{3}{8}\)
13. Exercise: States of matter
{TOPIC 1: Intermolecular Forces, Gas Laws and Ideal Gas Equation}
1. Which of the following is not a type of van der Waal's forces?
(a) Dipole - dipole forces
(b) Dipole - induced dipole forces
(c) Ion - dipole forces
(d) London forces
2. Gas equation \(P V=n R T\) is obeyed by
(a) only isothermal process
(b) only adiabatic process
(c) both (a) and (b)
(d) none of these
3. Air at sea level is dense. This is a practical application of
(a) Boyle's law
(b) Charle's law
(c) Kelvin's law
(d) Brown's law
4. "Equal volumes of all gases at the same temperature and pressure contain equal number of particles." This statement is a direct consequence of :
(a) Perfect gas law
(b) Avogadro's law
(c) Charle's law
(d) Boyle's law
5. A gas in an open container is heated from \(27^{\circ} \mathrm{C}\) to \(127^{\circ} \mathrm{C}\). The fraction of the original amount of the gas remaining in the container will be
(a) \(3 / 4\)
(b) \(1 / 2\)
(c) \(1 / 4\)
(d) \(1 / 8\)
6. The pressure exerted by \(6.0 \mathrm{~g}\) of methane gas in a \(0.03 \mathrm{~m} 3\) vessel at \(129^{\circ} \mathrm{C}\) is (Atomic masses : \(\mathrm{C}=\) \(12.01, \mathrm{H}=1.01\) and \(\mathrm{R}=8.314 \mathrm{JK}^{-1} \mathrm{~mol}^{-1}\) )
(a) \(31684 \mathrm{~Pa}\)
(b) \(215216 \mathrm{~Pa}\)
(c) \(13409 \mathrm{~Pa}\)
(d) \(41648 \mathrm{~Pa}\)
7. Two flasks \(A\) and \(\mathrm{B}\) of \(500 \mathrm{~mL}\) each are respectively filled with \(\mathrm{O}_{2}\) and \(\mathrm{SO}_{2}\) at \(300 \mathrm{~K}\) and \(1 \mathrm{~atm}\) pressure. The flasks will contain
(a) the same number of atoms
(b) the same number of molecules
(c) more number of moles of molecules in flask A as compared to flask B
(d) the same amount of gases
8. Dalton's law of partial pressure will not apply to which of the following mixture of gases
(a) \(\mathrm{H}_{2}\) and \(\mathrm{SO}_{2}\)
(b) \(\mathrm{H}_{2}\) and \(\mathrm{Cl}_{2}\)
(c) \(\mathrm{H}_{2}\) and \(\mathrm{CO}_{2}\)
(d) \(\mathrm{CO}_{2}\) and \(\mathrm{Cl}_{2}\)
9. Dipole-induced dipole interactions are present in which of the following pairs :
(a) \(\mathrm{Cl}_{2}\) and \(\mathrm{CCl}_{4}\)
(b) \(\mathrm{HCl}\) and \(\mathrm{He}\) atoms
(c) \(\mathrm{SiF}_{4}\) and \(\mathrm{He}\) atoms
(d) \(\mathrm{H}_{2} \mathrm{O}\) and alcohol
10. The pressure of sodium vapour in a \(1.0 \mathrm{~L}\) container is 10 torr at \(1000{ }^{\circ} \mathrm{C}\). How many atoms are in the container?
(a) \(9.7 \times 10^{17}\)
(b) \(7.6 \times 10^{19}\)
(c) \(4.2 \times 10^{17}\)
(d) \(9.7 \times 10^{19}\)
11. The atmospheric pressure on Mars is \(0.61 \mathrm{kPa}\). What is the pressure in mm Hg?
(a) 0.63
(b) 4.6
(c) 6.3
(d) 3.2
12. \(56 \mathrm{~g}\) of nitrogen and \(96 \mathrm{~g}\) of oxygen are mixed isothermaly and at a total pressure of \(10 \mathrm{~atm}\). The partial pressures of oxygen and nitrogen (in atm) are respectively:
(a) 4, 6
(b) 5,5
(c) 2,8
(d) 6, 4
13. What is the ratio of diffusion rate of oxygen to hydrogen?
(a) \(1: 4\)
(b) \(4: 1\)
(c) \(1: 8\)
(d) \(8: 1\)
14. Which of following graph(s) represents Boyle's law
I.




(a) Only I
(b) II and IV
(c) I and III
(d) Only III
15. Pressure remaining the same, the volume of a given mass of an ideal gas increases for every degree centigrade rise in temperature by definite fraction of its volume at
(a) \(0^{\circ} \mathrm{C}\)
(b) its critical temperature
(c) absolute zero
(d) its Boyle's temperature
16. The ratio of the rate of diffusion of helium and methane under identical condition of pressure and temperature will be
(a) 4
(b) 2
(c) 1
(d) 0.5
17. A gas diffuses \(1 / 5\) times as fast as hydrogen. Its molecular weight is
(a) 50
(b) 25
(c) \(25 \sqrt{2}\)
(d) \(50 \sqrt{2}\)
18. Which of the following are arranged in the correct order?
I. Gas \(>\) Liquid \(>\) Solid (Thermal energy)
II. Solid \(>\) Liquid \(>\) Gas (Intermolecular force)
Select the correct option.
(a) I only
(b) II only
(c) Both I and II
(d) None of these
19. At N.T.P the volume of a gas is found to be \(273 \mathrm{~mL}\). What will be the volume of this gas at \(600 \mathrm{~mm}\) of \(\mathrm{Hg}\) and \(273^{\circ} \mathrm{C}\) ?
(a) \(391.8 \mathrm{~mL}\) (b) \(380 \mathrm{~mL}\)
(c) \(691.6 \mathrm{~mL}\)
(d) \(750 \mathrm{Ml}\)
20. If three unreactive gases having partial pressures \(P_{A}, P_{B}\) and \(P_{C}\) and their moles are 1,2 and 3 respectively then their total pressure will be
(a) \(\mathrm{P}=\mathrm{P}_{\mathrm{A}}+\mathrm{P}_{\mathrm{B}}+\mathrm{P}_{\mathrm{C}}\)
(b) \(\mathrm{P}=\frac{\mathrm{P}_{\mathrm{A}}+\mathrm{P}_{\mathrm{B}}+\mathrm{P}_{\mathrm{C}}}{6}\)
(c) \(\mathrm{P}=\frac{\sqrt{\mathrm{P}_{\mathrm{A}}+\mathrm{P}_{\mathrm{B}}+\mathrm{P}_{\mathrm{C}}}}{3}\)
(d) None of these
21. For 1 mol of an ideal gas at a constant temperature \(T\), the plot of \((\log P)\) against \((\log V)\) is a \((P\) : Pressure, \(V\) : Volume)
(a) Straight line parallel to \(x\)-axis.
(b) Straight line with a negative slope.
(c) Curve starting at origin.
(d) Straight line passing through origin.
22. What is the value of \(\mathrm{X}\) in \({ }^{\circ} \mathrm{C}\) for given volume vs temperature curve ?

(a) \(0^{\circ} \mathrm{C}\)
(b) \(273.15^{\circ} \mathrm{C}\)
(c) \(-273.15^{\circ} \mathrm{C}\)
(d) \(300^{\circ} \mathrm{C}\)
23. \(4.4 \mathrm{~g}\) of a gas at STP occupies a volume of \(2.24 \mathrm{~L}\), the gas can be
(a) \(\mathrm{O}_{2}\)
(b) \(\mathrm{CO}\)
(c) \(\mathrm{NO}_{2}\)
(d) \(\mathrm{CO}_{2}\)
24. A certain gas takes three times as long to effuse out as helium. Its molecular mass will be :
(a) \(27 \mathrm{u}\)
(b) \(36 \mathrm{u}\)
(c) \(64 \mathrm{u}\)
(d) \(9 \mathrm{u}\)
25. The correct value of the gas constant ' \(R\) ' is close to :
(a) 0.082 litre-atmosphere \(\mathrm{K}\)
(b) 0.082 litre-atmosphere \(\mathrm{K}^{-1} \mathrm{~mol}^{-1}\)
(c) 0.082 litre - atmosphere \(^{-1} \mathrm{~K} \mathrm{~mol}^{-1}\)
(d) 0.082 litre -1 atmosphere \(^{-1} \mathrm{~K}\) mol
26. \(500 \mathrm{~mL}\) of air at \(760 \mathrm{~mm}\) pressure were compressed to \(200 \mathrm{~mL}\). If the temperature remains constant, what will be the pressure after compression?
(a) \(1800 \mathrm{~mm}\) (b) \(1900 \mathrm{~mm}\)
(c) \(2000 \mathrm{~mm}\)
(d) \(1500 \mathrm{~mm}\)
27. At a pressure of 760 torr and temperature of \(273.15 \mathrm{~K}\), the indicated volume of which system is not consistent with the observation
(a) \(14 \mathrm{~g}\) of \(\mathrm{N}_{2}+16 \mathrm{~g}\) of \(\mathrm{O}_{2} ;\) Volume \(=22.4 \mathrm{~L}\)
(b) \(4 \mathrm{~g}\) of \(\mathrm{He}+44 \mathrm{~g}\) of \(\mathrm{CO}_{2} ;\) Volume \(=44.8 \mathrm{~L}\)
(c) \(7 \mathrm{~g}\) of \(\mathrm{N}_{2}+36 \mathrm{~g}\) of \(\mathrm{O}_{3}\); Volume \(=22.4 \mathrm{~L}\)
(d) \(17 \mathrm{~g}\) of \(\mathrm{NH}_{3}+36.5 \mathrm{~g}\) of \(\mathrm{HCl}\), Volume \(=44.8 \mathrm{~L}\)
28. One mole of gas A and three moles of a gas B are placed in flask of volume 100 litres at \(27^{\circ} \mathrm{C}\). Calculate the total partial pressure of the gases in the mixture.
(a) \(1.0 \mathrm{~atm}\). (b) \(0.9 \mathrm{~atm}\)
(c) \(0.985 \mathrm{~atm}\)
(d) \(10.850 \mathrm{~atm}\).
29. Which of the following volume (V) - temperature (T) plots represents the behaviour of one mole of an ideal gas at one atmospheric pressure ?
(a)



30. A mixture of \(16 \mathrm{~g} \mathrm{CH}_{4}\) and \(64 \mathrm{~g}\) of \(\mathrm{O}_{2}\) is ignited in a sealed bulb of \(3 \mathrm{~L}\) and then cooled to \(27{ }^{\circ} \mathrm{C}\). The pressure in the bulb will be
(a) \(0.82 \mathrm{~atm}\)
(b) \(8.2 \mathrm{~atm}\)
(c) \(24.6 \mathrm{~atm}\)
(d) \(2.46 \mathrm{~atm}\)
\section{TOPIC 2: Kinetic Theory of Gases and Molecular Speeds}
31. The ratio between most probable velocity, mean velocity and r.m.s velocity is :
(a) \(\sqrt{2}: \sqrt{8 / \pi}: \sqrt{3}\)
(b) \(\sqrt{2}: \sqrt{3}: \sqrt{8 / \pi}\)
(c) \(1: 2: 3\)
(d) \(1: \sqrt{2}: \sqrt{3}\)
32. As the temperature is raised from \(20^{\circ} \mathrm{C}\) to \(40^{\circ} \mathrm{C}\), the average kinetic energy of neon atoms changes by a factor of which of the following ?
(a) \(\frac{313}{293}\)
(b) \(\sqrt{(313 / 293)}\)
(c) \(1 / 2\)
(d) 2
33. Boyle's law, according to kinetic equation can be expressed as
(a) \(\mathrm{PV}=\mathrm{KT}\)
(b) \(\mathrm{PV}=\mathrm{RT}\)
(c) \(\mathrm{PV}=\frac{3}{2} \mathrm{kT}\)
(d) \(P V=\frac{2}{3} \mathrm{kT}\)
34. The r.m.s velocity of \(\mathrm{CO}_{2}\) at temperature \(\mathrm{T}\) (in kelvin) is \(x \mathrm{cms}^{-1}\). At what temperature (in kelvin) the r.m.s. velocity of nitrous oxide would be \(4 \mathrm{x} \mathrm{cms}^{-1}\) ?
(a) \(16 \mathrm{~T}\)
(b) \(2 \mathrm{~T}\)
(c) \(4 \mathrm{~T}\)
(d) \(32 \mathrm{~T}\)
35. The molecular velocities of two gases at the same temperature are \(\mathbf{u}_{1}\) and \(\mathbf{u}_{2}\) and their masses are \(\mathrm{m}_{1}\) and \(\mathrm{m}_{2}\) respectively. Which of the following expressions are correct?
1) \(\frac{\mathrm{m}_{1}}{\mathrm{u}_{1}^{2}}=\frac{\mathrm{m}_{2}}{\mathrm{u}_{2}^{2}}\)
2) \(\mathrm{m}_{1} \mathrm{u}_{1}=\mathrm{m}_{2} \mathrm{u}_{2}\)
3) \(\frac{m_{1}}{u_{1}}=\frac{m_{2}}{u_{2}}\)
4) \(\mathrm{m}_{1} \mathrm{u}_{1}^{2}=\mathrm{m}_{2} \mathrm{u}_{2}^{2}\)
36. Kinetic theory of gases proves
(a) only Boyle's law
(b) only Charles' law
(c) only Avogadro's law
(d) All of these
37. The root mean square velocity of an ideal gas at constant pressure varies with density \((d)\) as
(a) \(d^{2}\)
(b) \(d\)
(c) \(\sqrt{\mathrm{d}}\)
(d) \(1 / \sqrt{\mathrm{d}}\)
38. Which one of the following is the wrong assumption of kinetic theory of gases ?
(a) Momentum and energy always remain conserved.
(b) Pressure is the result of elastic collision of molecules with the container's wall.
(c) Molecules are separated by great distances compared to their sizes.
(d) All the molecules move in straight line between collision and with same velocity.
39. Consider three one-litre flasks labelled A, B and C filled with the gases \(\mathrm{NO} \mathrm{NO}_{2}\), and \(\mathrm{N}_{2} \mathrm{O}\), respectively, each at \(1 \mathrm{~atm}\) and \(273 \mathrm{~K}\). In which flask do the molecules have the highest average kinetic energy?
(a) Flask C
(b) All are the same
(c) Flask A
(d) None
40. Which of the following change is observed occurs when a substance \(\mathrm{X}\) is converted from liquid to vapour phase at the standard boiling point?
I. Potential energy of the system decreases
II. The distance between molecules increases
III. The average kinetic energy of the molecules in both phases are equal
(a) I only
(b) II only
(c) III only
(d) II and III only
41. If \(\mathrm{u}_{\mathrm{rms}}\) of a gas is \(30 \mathrm{R}^{1 / 2} \mathrm{~ms}^{-1}\) at \(27{ }^{\circ} \mathrm{C}\) then molar mass of gas is:
(a) \(0.02 \mathrm{~kg} / \mathrm{mol}\)
(b) \(0.001 \mathrm{~kg} / \mathrm{mol}\)
(c) \(0.003 \mathrm{~kg} / \mathrm{mol}\)
(d) \(1 \mathrm{~kg} / \mathrm{mol}\)
42. Consider a real gas placed in a container. If the intermolecular attractions are supposed to disappear suddenly which of the following would happen?
(a) Pressure decreases (b) Pressure increases (c) Pressure remains unchanged (d) Gas collapse
43. The pressure of a real gas is less than the pressure of an ideal gas because of :
(a) increase in number of collisions
(b) finite size of molecule
(c) increase in KE of molecules
(d) intermolecular forces of attraction
44. At what temperature the rms velocity of \(\mathrm{SO}_{2}\) be same as that of \(\mathrm{O}_{2}\) at \(303 \mathrm{~K}\) ?
(a) \(273 \mathrm{~K}\)
(b) \(606 \mathrm{~K}\)
(c) \(303 \mathrm{~K}\)
(d) \(403 \mathrm{~K}\)
45. A closed flask contains water in all its three states solid, liquid and vapour at \(0{ }^{\circ} \mathrm{C}\). In this situation, the average kinetic energy of water molecules will be
(a) equal in all the three states
(b) the greatest in vapour state
(c) the greatest in the liquid state
(d) the greatest in the solid state
\section{TOPIC 3: Van der Waal's Equation and Liquefaction of Gases}
46. The compressibility factor for \(\mathrm{H} 2\) and He is usually :
(a) \(\mathrm{Z}>1\)
(b) \(\mathrm{Z}=1\)
(c) \(\mathrm{Z}<1\)
(d) Either of these
47. It \(V\) is the volume of one molecule of gas under given conditions, the van der Waal's constant \(b\) is
(a) \(4 \mathrm{~V}\)
(b) \(\frac{4 \mathrm{~V}}{\mathrm{~N}_{0}}\)
3) \(\frac{\mathrm{N}_{0}}{4 \mathrm{~V}}\)
4) \(4 \mathrm{VN}_{0}\)
48. The units of constant ' \(a\) ' in van der Waal's equation is
(a) \(\mathrm{dm}^{6} \mathrm{~atm} \mathrm{~mol}^{-2}\)
(b) \(\mathrm{dm}^{3} \mathrm{~atm} \mathrm{~mol}^{-1}\)
(c) \(\mathrm{dm}\) atm \(\mathrm{mol}^{-1}\)
(d) atm \(\mathrm{mol}^{-1}\) 49. Maximum deviation from ideal gas is expected from :
(a) \(\mathrm{N}_{2}(\mathrm{~g})\)
(b) \(\mathrm{CH}_{4}(\mathrm{~g})\)
(c) \(\mathrm{NH}_{3}(\mathrm{~g})\)
(d) \(\mathrm{H}_{2}(\mathrm{~g})\)
50. At which one of the following temperature-pressure conditions the deviation of a gas from ideal behaviour is expected to be minimum?
(a) \(350 \mathrm{~K}\) and \(3 \mathrm{~atm}\).
(b) \(550 \mathrm{~K}\) and \(1 \mathrm{~atm}\).
(c) \(250 \mathrm{~K}\) and \(4 \mathrm{~atm}\).
(d) \(450 \mathrm{~K}\) and \(2 \mathrm{~atm}\).
51. In van der Waal's equation of state for a non-ideal gas, the term that accounts for intermolecular forces is
1) \((\mathrm{V}-\mathrm{b})\)
2)RT
3) \(\left(\mathrm{P}+\frac{\alpha}{\mathrm{V}^{2}}\right)\)
4) \((\mathrm{RT})^{-1}\)
52. In van der Waal's equation of state of the gas law, the constant ' \(b\) ' is a measure of
(a) volume occupied by the molecules
(b) intermolecular attraction
(c) intermolecular repulsions
(d) intermolecular collisions per unit volume
53. Positive deviation from ideal behaviour takes place because of
(a) molecular interaction between atoms and \(P V / n R T>1\)
(b) molecular interaction between atoms and \(P V / n R T<1\)
(c) finite size of atoms and \(P V / n R T>1\)
(d) finite size of atoms and \(P V / n R T<1\)
54. The ratio of Boyle's temperature and critical temperature for a gas is :
1) \(\frac{8}{27}\)
2) \(\frac{27}{8}\)
3) \(\frac{1}{2}\)
4) \(\frac{2}{1}\)
55. The compressibility factor for a real gas at high pressure is:
1) 1
2) \(1+\frac{\mathrm{Pb}}{\mathrm{RT}}\)
3) \(1-\frac{\mathrm{Pb}}{\mathrm{RT}}\)
4) \(1+\frac{\mathrm{RT}}{\mathrm{Pb}}\)
56. The van der Waal's constant ' \(a\) ' for four gases \(P, Q, R\) and \(S\) are 4.17, 3.59, 6.71 and \(3.8 \mathrm{~atm} \mathrm{~L}^{2} \mathrm{~mol}^{-2}\) respectively. Therefore, the ascending order of their liquefaction is
(a) \(\mathrm{R}<\mathrm{P}<\mathrm{S}<\mathrm{Q}\)
(b) \(\mathrm{Q}<\mathrm{S}<\mathrm{R}<\mathrm{P}\)
(c) \(\mathrm{Q}<\mathrm{S}<\mathrm{P}<\mathrm{R}\)
(d) \(\mathrm{R}<\mathrm{P}<\mathrm{Q}<\mathrm{S}\)
\section{TOPIC 4: Liquid State}
57. The correct order of viscosity of the following liquids will be
(a) Water \(<\) methyl alcohol \(<\) dimethyl ether \(<\) glycerol
(b) methyl alcohol < glycerol < water \(<\) dimethyl ether
(c) dimethyl ether \(<\) methyl alcohol \(<\) water \(<\) glycerol
(d) glycerol \(<\) dimethyl ether \(<\) water \(<\) methyl alcohol
58. Which among the following has lowest surface tension ?
(a) Hexane
(b) Water
(c) \(\mathrm{CH}_{3} \mathrm{OH}\)
(d) \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{OH}\)
59. The liquid which has the highest rate of evaporation is
(a) petrol
(b) nail-polish remover (c) water
(d) alcohol
60. A drop of oil is placed on the surface of water. Which of the following statement is correct ?
(a) It will remain on it as a sphere
(b) It will spread as a thin layer
(c) It will be partly as spherical droplets and partly as thin film
(d) It will float as a distorted drop on the water surface
14. Solid States
1. Which of the following is an evample of covalent solid?
(a) Silicon carboide
(b) \(\mathrm{BaSO}_4\)
(c) Solid \(\mathrm{CO}_3\)
(d) Iodine
2. The number of basic types of unit cells among the erystal is
(a) Eight
(b) Fourteen
(c) Seven
(d) Ten
3. The number of atoms per unit cell in a body centered cubic arrangement is
(a) I
(b) 2
(c) 4
(d) 6
4. Which of the following is a molecular solid?
(a) \(\mathrm{MgO}\)
(b) \(\mathrm{Ag} \mathrm{Cl}\)
(c) \(\mathrm{CO}_2\)
(d) \(\mathrm{Pd}\)
5. The coordination number in a ccp arrangement of atoms is
(a) 12
(b) 8
(c) 6
(d) 4
6. The unit cell with crystallographic dimensions a \(=\mathrm{b} \neq c, a=\beta=\gamma=90\)
(a) Cubic
(b) Hexagonal
(c) Tetragonal
(d) Orthorhombic
7. Which one has highest melting point?
(a) ionic crystal
(b) Molecular crystal
(c) Covalent crystal
(d) Metallic crystal
8. Which of the following is not the property of crystalline solids?
(a) definite geometry
(b) sharp melting point
(c) isotropy
(d) anisotropy
9. In CsCl structure, the coordination number of \(\mathrm{Cs}^{+}\) and \(\mathrm{Cl}^{-1}\) ions are respectively
(a) 8,8
(b) 8,6
(c) 6,8
(d) 6,6
10. Which of the following transition metal oxides is diamagnetic?
(a) \(\mathrm{VO}\)
(b) \(\mathrm{V}_2 \mathrm{O}_3\)
(c) \(\mathrm{YO}_2\)
(d) \(\mathrm{V}_2 \mathrm{O}_5\)
11. An example of ferroelectric is
(a) Quartz
(b) Barium titanate
(c) Tourmaline
(d) Leed zirconate
12. A metal has face centered cubic arrangement. If length of the edge of the cell is \(\times \mathrm{pm}\) and \(\mathrm{M}\) is its atomic mass, then density will be equal to \(\left(\mathrm{N}_{\mathrm{n}}\right.\) is Avogadro number)
(a) \(\frac{\mathrm{M}}{\mathrm{x}^3 \times \mathrm{N}_0} \mathrm{gcm}^{-3}\)
(b) \(\frac{4 \mathrm{M}}{\mathrm{x}^2 \times \mathrm{N}_0} \mathrm{~g} \mathrm{~cm}^{-3}\)
(c) \(\frac{4 \mathrm{M}}{\mathrm{x}^3 \times \mathrm{N}_0} g \mathrm{gcm}^{-3}\)
(d) \(\frac{M}{4 x^3 \times N_0} g^{-1} \mathrm{~cm}^{-3}\)
13. The basic unit in pyrosillicates is
(a) \(\mathrm{SiO}_4{ }^{2-}\) (b) \(\left[\mathrm{Si}_3 \mathrm{O}_4\right]^{b-}\) (c) \(\left[\mathrm{SuO}_3^{2-}\right]_4\) (d) \(\left[\mathrm{Si}_2 \mathrm{O}_4\right]^{b-}\)
14. The arrangement in an ideal crystal ( \(\mathrm{AB}\) and a defect structured crystal is shown below \(\mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{+}\)
\(\mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{-} \quad \mathrm{A}^{+} \mathrm{B}^{-}\)
\(\mathrm{B}^{-} \mathrm{A}^{+} \quad \mathrm{B}^{-} \mathrm{A}^{+} \quad \mathrm{B}^{-} \mathrm{A}^{+} \quad \mathrm{B}^{-} \mathbf{A}^{+}\)
(Ideal crystal) (Defect structure) This illustrates the example of
(a) Frenkel defect
(b) Schottky defect
(c) Metal excess defect
(d) Metal deficient defect
15. Which of the following transition metal oxides is not paramagnetic?
(a) \(\mathrm{TiO}\)
(b) \(\mathrm{CuO}\)
(c) \(\mathrm{VO}\)
(d) \(\mathrm{TiO}_2\)
16. If the alignment of magnetic moments in a substance is in a compensatiory way so as to give zero net magnetic moment, then the substance is said to be
(a) Ferromagnetic
(b) Anti-ferromagnetic
(c) Ferrimagnetic
(d) Diamagnetic
17. The number of oxygen atoms shared between silicon atoms in \(\mathrm{Si}_3 \mathrm{O}_9^{-}\)- ion is
(a) One
(b) Two
(c) Three
(d) \(\operatorname{Six}\)
18. When atoms are placed at the corners of all 12 edges of a cube, the number of atoms present per unit cell is
(a) 1
(b) 2
(c) 4
(d) 6
19. A compound formed by elements \(A\) and \(B\) has a cubic structure in which \(A\) atoms are at the corners of the cube and \(B\) atoms are at the face centres. The formula of the compound is
(a) \(\mathrm{AB}_3\)
\(\begin{array}{ll}\text { (b) } \mathrm{A}_2 \mathrm{~B}_3 & \text { (c) } \mathrm{A}_2 \mathrm{~B}\end{array}\)
(d) \(\mathrm{A}_4 \mathrm{~B}_3\)
20. One unit cell of \(\mathrm{NaCl}\) contains
(a) \(1 \mathrm{Na}^{+}\)and \(1 \mathrm{Cl}^{-}\)
(b) \(2 \mathrm{Na}^{+}\)and \(2 \mathrm{Cl}^{-}\)
(c) \(4 \mathrm{Na}^{+}\)and \(4 \mathrm{Cl}^{-}\)
(d) \(12 \mathrm{Na}^{+}\)and \(12 \mathrm{Cl}^{-}\)
21. An example of antiferroelectric substance is
(a) \(\mathrm{PbZrO}_3\) (b) \(\mathrm{BaTiO}_3\) (c) \(\mathrm{Fe}_3 \mathrm{O}_4\)
(d) \(\mathrm{KH}_2 \mathrm{PO}_4\)
22. Which of the following is an example of three dimensional silicates?
(a) quartz (b) talc
(c) asbestos (d) beryl
23. The number of \(\mathrm{NaCl}\) formula units in a unit cell of sodium chloride is
(a) 1
(b) 2
(c) 4
(d) 8
24. The most unsymmetrical unit cell is
(a) Monoclinic
(b) Triclinic
(c) Orthorhombic
(d) Tetragonal
25. Asbestos is an example of
(a) Island chain silicates
(b) Orthosilicates
(c) Chain silicates
(d) Cyclic silicates
26. Body centered cubic lattice has coordination number
(a) 6
(b) 8
(c) 10
(d) 12
27. Crystals can be described into basic crystal habits
(a) 7
(b) 4
(c) 14
(d) 3
28. Which of the following is having hexagonal close packed crystal structure?
(a) \(\mathrm{NaCl}\)
(b) \(\mathrm{CsCl}\)
(c) \(\mathrm{Zn}\)
(d) \(\mathrm{RbCl}\)
29. A metallic element crystallizes into a lattice containing a sequence of layers \(A B A B A B\) Any packing of spheres leaves out voids in the lattice. What percentage by volume of this lattice is empty space?
(a) \(74 \%\)
(b) \(20 \%\)
(c) \(50 \%\)
(d) none of these
30. A metal crystallizes in cubic close packed (cep). The atomic radius of metal is \(144 \mathrm{pm}\) and its atomic mass is 197 a.m.u. Its density is
(a) \(19.4 \mathrm{~g} \mathrm{~cm}^{-3}\)
(b) \(1.94 \mathrm{~g} \mathrm{~cm}^{-3}\)
(c) \(29.4 \mathrm{~g} \mathrm{~cm}^{-3}\)
(d) \(2.94 \mathrm{~g} \mathrm{~cm}^{-3}\)
31. In a close packing of atoms \(A\) of radius \(r_{\text {the }}\) the radius of atom \(B\) that can be fitted in tetrahedral void is
(a) \(0.225 \mathrm{r}_{\mathrm{a}}\) (b) \(0.155 \mathrm{r}_{\mathrm{a}}\) (c) \(0.414 \mathrm{r}_2\) (d) \(0.732 \mathrm{r}_3\)
32. The edge length of a cube is \(520 \mathrm{pm}\). Its body diagonal would be
(a) \(800.62 \mathrm{pm}\)
(b) \(900.64 \mathrm{pm}\)
(c) \(735.28 \mathrm{pm}\)
(d) \(520.0 \mathrm{pm}\)
33. Which of the following dimensions represent triclinic unit cell?
(a) \(a=b=c, \alpha=\beta=\gamma=90^{\circ}\)
(b) \(\mathrm{a}=\mathrm{b} \neq \mathrm{c}, \alpha=\beta=\gamma=90^0\)
(c) \(a \neq b \neq c, \alpha=\beta=\gamma=90^{\circ}\)
(d) \(a \neq b \neq c, \alpha \neq \beta \neq \gamma=90^{\circ}\)
34. In a primitive cubic lattice, the percentage of void volume is
(a) \(52.36 \%\) (b) \(25.95 \%\) (c) \(74.05 \%\) (d) \(47.64 \%\)
35. A mineral having the formula \(\mathbf{A B}\), crystallized in cubic closed packed lattice with the atoms \(A\) occupying lattice points. The co-ordination number of \(A\) atoms, that of \(B\) atoms and the fraction of the tetrahedral sites occupied by \(B\) atoms are
(a) \(2,6,75 \%\)
(b) \(8,4,100 \%\)
(c) \(3,1,25 \%\)
(d) \(6,6,50 \%\)
36. \(\mathrm{Fe}_3 \mathrm{O}_4\) is ferrimagnetic at room temperature but at \(850 \mathrm{~K}\) it becomes
(a) Diamagnetic
(b) Ferromagnetic
(c) Non-magnetic
(d) Paramagnetic
37. A substance \(A_x B_y\) crystallizes in a face centered cubic ( \(\mathrm{FCC})\) lattice in which atoms 'A' occupy each corner of the cube and atoms 'B' occupy the centers of each face of the cube. Identify the correct composition of the substance \(A_x B_y\)
(a) \(\mathrm{AB}_3\)
(b) \(\mathrm{A}_4 \mathrm{~B}_3^3\)
(c) \(\mathrm{A}_3 \mathrm{~B}^3\)
(d) Composition cannot be specified
38. Fraction of total volume occupied by atoms in a simple cubic cell is
(a) \(\frac{\pi}{2}\)
(b) \(\frac{\sqrt{3 \pi}}{8}\)
(c) \(\frac{\sqrt{2} \pi}{6}\)
(d) \(\frac{\pi}{6}\)
39. In a cubic crystal anions are arrangement in fcc arrangement and the cations occupy all the octahedral voids and half the tetrahedral voids. The ratio of the cations and anions in the crystal is
(a) \(1: 1\)
(b) \(2: 1\)
(c) \(1: 2\)
(d) \(3: 2\)
40. The number of atoms in \(100 \mathrm{~g}\) of the fcc crystal with density \(10 \mathrm{~g} \mathrm{~cm}^{-3}\) and edge length of \(200 \mathrm{pm}\) is
(a) \(1 \times 10^{25}\)
(b) \(5 \times 10^{24}\)
(c) \(5 \times 10^{26}\)
(d) \(5 \times 10^{20}\)
41. In a structure, A atoms have fcc arrangement. B atoms occupy all the tetrahedral slites and \(\mathrm{C}\) atoms occupy half the octahedral sides. The formula ofthe compound is
42. Schottky defect in crystal is observed when
(a) unequal number of cations and anions are missing from the lattice
(b) equal number of cations and anions are missing from the lattice
(c) an ion leaves its normal site and occpies an intestitial site
(d) density of the crystal is increased
43. When molten zinc is cooled to solid state, it assumes hcp structure. Then the number of nearest neighbours of zinc atom will be
(a) 4
(b) 6
(c) 8
(d) 12
44. The pykno meter density of sodium chloride crystal is \(2.165 \times 10^3 \mathrm{~kg} \mathrm{~m}^{-3}\) while its X-ray density \(2.178 \times 10^3 \mathrm{~kg} \mathrm{~m}^{-3}\).
(a) 5.96
(b) \(5.96 \times 10^{-2}\)
(c) \(5.96 \times 10^{-1}\)
(d) \(5.96 \times 10^{-3}\)
45. CsBr crystallizes in a body centered cubic lattice. The unit cell lengths is \(436.6 \mathrm{pm}\). Given that the atomic mass of \(\mathrm{Cs}=133\) and that of \(\mathrm{Br}=80 \mathrm{amu}\) and Avogadro number being \(6.02 \times 10^{23} \mathrm{~mol}^{-1}\). The density of \(\mathrm{CsBr}\) is
(a) \(0.425 \mathrm{~g} \mathrm{~cm}^{-3}\)
(b) \(8.25 \mathrm{~g} \mathrm{~cm}^{-3}\)
(c) \(4.25 \mathrm{~g} \mathrm{~cm}^{-3}\)
(d) \(42.5 \mathrm{~g} \mathrm{~cm}^{-3}\)
46. In a face centered cubic lattice, a unit cell is shared equally by how many unit cells?
(a) 2
(b) 4
(c) 6
(d) 8
47. If \(\mathrm{NaCl}\) is doped with \(10^{-4} \mathrm{~mol} \%\) of \(\mathrm{SrCl}_2\), the concentration of cation vacancies will be ( \(\mathrm{NA}=\) \(6.022 \times 10^{23} \mathrm{~mol}^{-1}\) )
(a) \(6.02 \times 10^{16} \mathrm{~mol}^{-1}\)
(b) \(6.02 \times 10^{17} \mathrm{~mol}^{-1}\)
(c) \(6.02 \times 10^{14} \mathrm{~mol}^{-1}\)
(d) \(6.02 \times 10^{15} \mathrm{~mol}^{-1}\)
48. the fraction of total volume occupied by the atoms present in a simple cube is
(a) \(\frac{\pi}{3 \sqrt{2}}\)
(b) \(\frac{\pi}{4 \sqrt{2}}\)
(c) \(\frac{\pi}{4}\)
(d) \(\frac{\pi}{6}\)
49. How many unit cells are present in a cube shaped ideal crystal of \(\mathrm{NaCl}\) of mass \(1.0 \mathrm{~g}\) ?
(a) \(5.14 \times 10^{21}\)
(b) \(1.28 \times 10^{21}\)
(c) \(1.71 \times 10^{21}\)
(d) \(2.57 \times 10^{21}\)
50. In which of the following crystals alternate tetrahedral voids are occupied?
(a) \(\mathrm{NaCl}\)
(b) \(\mathrm{ZnS}\)
(c) \(\mathrm{CaF}_2\)
(d) \(\mathrm{Na}_2 \mathrm{O}\)

15. Solutions and colligative properties
1. A molal solution is one that contains one mole of solute in
(a) one litre of the solvent
(b) 1000 g of the solvent
(c) one litre of the solution
(d) 22.4 litres of solution.
2. Which of the following is independent of temperature?
(a) normality
(b) molarity
(c) molality
(d) formality
3. When \(\mathrm{NaCl}\) is added to water.
(a) freezing point is raised
(b) boiling point is depressed
(c) freezing point does not change
(d) boiling point is raised
4. Which of the following 0.1 \(\mathrm{M}\) aqueous solutions will exert highest osmotic pressure?
(a) \(\mathrm{NaCl}\)
(b) \(\mathrm{BaCl}_2\)
(c) \(\mathrm{MgSO}_4\)
(d) \(\mathrm{Al}\left(\mathrm{SO}_4\right)_3\)
5. According to the Raoult's law, the relative lowering of vapor pressure is equal to the
(a) mole fraction of solvent
(b) mole fraction of solute
(c) independent of mole fraction of solute
(d) molality of solution
6. Partial pressure of solvent in solution of nonvolatile solute is given by equation,
(a) \(\mathrm{p}=\mathrm{x}_2 \mathrm{P}^0\)
(b) \(\mathrm{p}^6=x p\)
(c) \(p=X_1 P^0\)
(d) \(\mathrm{P}^6=\mathrm{x}_1 \mathrm{P}\)
7. When partial pressure of solvent in solution of non-volatile solute is plotted against its mole fraction, nature of graph is
(a) a straight line passing through origin
(b) a straight line parallel to mole fraction of solvent
(c) a straight line parallel to vapor pressure of solvent
(d) a straight line intersecting vapor pressure axis.
8. Lowering of vapor pressure of solution
(a) is a property of solute
(b) is a property of solute as well as solvent
(c) is a property of solvent
(d) is a colligative property
9. Molal elevation constant is elevation in ceiling point produced by
(a) \(1 \mathrm{~g}\) of solute in \(100 \mathrm{~g}\) of solvent
(b) \(100 \mathrm{~g}\) of solute in \(1000 \mathrm{~g}\) of solvent
(c) 1 mole of solute in one litre of solvent
(d) 1 mole of solute in one \(\mathrm{kg}\) of solvent
10. The determination of molar mass from elevation in boiling point is called as
(a) cryoscopy
(b) osmometry
(c) ebullioscopy
(d) spectroscopy
11. Vapour pressure of solution of a non volatile solute is alvays
(a) equal to the vapour pressure of pure solvent
(b) higher than vapour pressure of pare solvent
(c) lower than vapour pressure of pure solvent
(d) constant
12. In osmasis
(a) solvent molecules pass from high
concentration of solute to low concentration.
(b) solvent molecules pass from a solution of low concentration of solute to a solution of high concentration of solute.
(c) solute molecules pass from low concentration to high concentration
(d) solute molecules pass from high concentration to low concentration.
13. The two solutions with same atmotic pressure are called
(a) isotonic
(b) isomeric
(c) hypotonic
(d) hypertonic
14. The values of gas constant and solution constant
(a) are different
(b) almost identical
(c) gas constant is greater than solution constant
(d) gas constant is smaller than solution constant
15. Abnormal molar mass is produced by
(a) association of solute
(b) dissociation of solute
(c) both association and dissociation of solute
(d) separation by semipermeable membrane
16. \(20 \mathrm{~g}\) of \(\mathrm{NaOH}\) (Molar mass \(=40 \mathrm{~g} \mathrm{~mol}^{-1}\) ) is dissolved in \(500 \mathrm{~cm}\) ? of water. Molality of resulting solution is
(a) \(0.1 \mathrm{~m}\)
(b) \(0.5 \mathrm{~m}\)
(c) \(1.5 \mathrm{~m}\)
(d) \(1.0 \mathrm{~m}\)
17. Molarity of solution depends on
(a) temperature
(b) nature of solute dissolved
(c) mass of solvent
(d) pressure
18. Which of the following aqueous solutions will have minimum elevation in boiling point?
(a) \(0.1 \mathrm{M} \mathrm{KCl}\)
(b) \(0.05 \mathrm{M} \mathrm{NaCl}\)
(c) \(1 \mathrm{MAIPO}_4\)
(d) \(0.1 \mathrm{M} \mathrm{MgSO}_4\)
19. Which of the following will have maximum depression in freezing point?
(a) \(0.5 \mathrm{M} \mathrm{Li}_2 \mathrm{SO}_4\)
(b) \(1 \mathrm{M} \mathrm{KCl}\)
(c) \(0.5 \mathrm{MAD2}\left(\mathrm{SO}_4\right)_3\)
(d) \(0.5 \mathrm{M} \mathrm{BaCl}_2\)
20. Relative vapour pressure lowering depends only on
(a) Mole fraction of solute
(b) Nature of solvent
(c) Nature of solute
(d) Nature of solute and solvent
21. If mass is expressed in gram then \(K_b\) is given by
(a) \(\frac{\mathrm{M}_2 \Delta \mathrm{T}_{\mathrm{b}} \times \mathrm{W}_2}{1000 \times \mathrm{W}_1}\)
(b) \(\frac{\mathrm{W}}{\Delta \mathrm{T}_{\mathrm{i}} \times \mathrm{W}_1 \times \mathrm{M}_2} \times 1000\)
(c) \(\frac{\mathrm{M}_2 \Delta \mathrm{T}_{\mathrm{b}} \times \mathrm{W}_1}{1000 \times \mathrm{W}_2}\)
(d) \(\frac{\mathrm{W}_2}{\mathrm{ST}_{\mathrm{b}} \times \mathrm{W}_2 \times \mathrm{M}_2} \times 1000\)
22. A solution is
(a) a mixture of two components
(b) a compound of two components
(c) a homogeneous mixture of two components
(d) all the above
23. A solution is \(0.25 \%\) by weight. The weight of solvent containing \(1.25 \mathrm{~g}\) - of solute would be
(a) \(500 \mathrm{~g}\).
(b) \(498.75 \mathrm{~g}\).
(c) \(50025 \mathrm{~g}\)
(d) \(501.25 \mathrm{~g}\).
24. Equal volumes of a \(10 \%\) solution (by weight of the solute \(\mathrm{A}\) and \(15 \%\) solution (by weight) of the solute \(B\) are mixed. The weight percent of \(A\) and \(B\) in the mixture would be respectively
(a) 5 and 7.5
(b) 10 and 15
(c) 15 and 10
(d) 20 and 30
25. \(1 \mathrm{dm}^3\) of water contains \(90 \mathrm{~g}\). of glucose. The mole fraction of glucose in the solution is
(a) 0.33
(b) 0.66
(c) \(0.5 / 56.05\)
(d) \(0.5 / 55.55\)
26. Two solutions \(A\) and \(B\) have same mole fractions of the solute. If \(1 \mathrm{dm}^3\) of \(\mathrm{A}\) is mixed with \(2 \mathrm{dm}^3\) of \(\mathrm{B}\), the mole fraction of the solute in the mixture would
(a) decrease
(b) increase
(c) remain unchanged
(d) change unpredictably
27. Which one of the following statements is true for a solution?
(a) Normality is always equal to molarity
(b) Normality is always less than molarity
(c) Molarity is always less than normality
(d) Molarity is either equal to or less than normality
28. For a solution of tartaric acid (HOOC.CHOH.CHOH.COOH) the molarity (M) and normality ( \(\mathrm{N})\) are related as
(a) \(\mathrm{N}=\mathrm{M} / 2\)
(b) \(2 \mathrm{M}=\mathrm{N}\)
(c) \(\mathrm{M}=\mathrm{N}\)
(d) \(\mathrm{M}>\mathrm{N}\)
29. Which one of the following is a colligative property?
(a) Boiling point
(b) Freezing point
(c) Vapour pressure
(d) Lowering of vapour pressure
30. For a solution, Vapour pressure of solution = Partial vapour pressure of solvent. This means that
(a) the solution is dilute
(b) the solute is non-volatile
(c) the solvent is volatile
(d) the solution is at room temperature
31. The plot of partial vapour pressure of solvent versus its mole fraction in the solution, at a constant temperature is
(a) a straight line
(b) a straight line parallel to one axis
(c) a straight line passing through the origin
(d) none of the above
32. The vapour pressures of pure solvent and solution are \(120 \mathrm{~mm} \mathrm{Hg}\) and \(108 \mathrm{~mm} \mathrm{Hg}\) respectively. The mole fraction of the solvent in the solution is
(a) 0.1
(b) 0.9
(c) \(120 / 108\)
(d) 1.08
33. In Ostwald and Walker's method for determining the lowering of vapour pressure, the loss in mass of solvent bulbs in \(\mathbf{5 0}\) times less than the gain in mass of \(\mathrm{CaCl}_2\) tubes. The vapour pressure of the solution would be (Given V.P. of pure solvent \(=100 \mathrm{~mm} \mathrm{Hg}\) )
(a) \(2 \mathrm{~mm} \mathrm{Hg}\)
(b) \(0.5 \mathrm{~mm} \mathrm{Hg}\)
(c) \(98 \mathrm{~mm} \mathrm{Hg}\)
(d) \(100 \mathrm{~mm} \mathrm{Hg}\)
34. Boiling point of a liquid under given conditions is the temperature at which its vapour pressure
(a) is equal to \(760 \mathrm{~mm} \mathrm{Hg}\)
(b) is equal to atmospheric pressure
(c) is equal to \(1 \mathrm{~atm}\).
(d) changes very rapidly
35. The elevation of boiling point is directly proportional to
(a) molality of the solution
(b) depression of freezing point in the same solution
(c) both these
(d) none of these
36. Which one of the following aqueous solutions will have the highest boiling point?
(a) \(0.1 \mathrm{M}\) urea
(b) \(30 \mathrm{~g}\). of glucose per \(\mathrm{dm}^3\)
(c) \(3.42 \mathrm{~g}\). of sucrose in \(100 \mathrm{ml}\)
(d) \(0.2 \mathrm{M}\) glucose
37. Which one of the following aqueous solutions will have same boiling point as that of \(0.2 \mathrm{M}\) aqueous solution of fructose?
(a) \(34.2 \mathrm{~g}\) sucrose per \(\mathrm{dm}^3\)
(b) \(36 \mathrm{~g}\). glucose in \(500 \mathrm{~cm}^3\)
(c) \(180 \mathrm{~g}\). glucose in \(10 \mathrm{dm}^3\)
(d) \(12 \mathrm{~g}\). urea per \(\mathrm{dm}^3\)
38. To determine the elevation of boiling point more accurately, the solvent used should have
(a) higher value of \(K_b\)
(b) lower value of \(\mathrm{K}_{\mathrm{b}}\)
(c) high mol, mass
(d) low mol. mass
39. Some statements are given below about depression of freezing point in a solution
(A) It is directly proportional to lowering of vapour pressure
(B) It is directly proportional to relative lowering of vapour pressure
(C) It is independent of the molarity of the solution
(D) It is directly proportional to the elevation of boiling point in the same solution
Among the above, the correct statements are
(a) only \(A\) and \(D\)
(b) A, C and D
(c) A, B and D
(d) all
40. The scale of the Beckmann's thermometer is only of \(5^{\circ}-6^{\circ} \mathrm{C}\) but the thermometer can be used over a wide range of temperature. This is due to
(a) its large length
(b) bigger size of the bulb
(c) large amount of mercury present
(d) the facility of adjusting the quantity of mercury in the bulb as per requirement
41. The values of \(K_t\) for solvents \(A, B, C\) and \(D\) are \(1.86,1.99,5.12\) and \(4.7 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\) respectively. The equimolal solutions of a solute in these solvents will have the freezing points in order of solvent as
(a) \(\mathrm{C}>\mathrm{D}>\mathrm{B}>\mathrm{A}\)
(b) A \(>\) C \(>\) D \(>\) B
(c) \(\mathrm{A}>\mathrm{B}>\mathrm{D}>\mathrm{C}\)
(d) \(\mathrm{C}>\mathrm{B}>\mathrm{A}>\mathrm{D}\)
42. Osmotic pressure is defined as
(a) the excess pressure which must be applied to a solution to stop osmosis
(b) the excess pressure which must be applied to a solution to increase its vapour pressure till it becomes equal to the vapour pressure of pure solvent
(c) the decrease in pressure on the pure solvent to decrease its vapour pressure till it becomes equal to the vapour pressure of solution
(d) all the above
43. The Van't Hoff factor for \(0.1 \mathrm{M} \mathrm{Ba}\left(\mathrm{NO}_3\right)_2\) solution is 2.74. The degree of dissociation is
(a) \(91.3 \%\)
(b) \(87 \%\)
(c) \(100 \%\)
(d) \(74 \%\)
44. A \(0.2 \mathrm{M}\) solution of a solute is isotonic with a solution containing
(a) 3.42 g. of sucrose in \(100 \mathrm{~cm}^3\)
(b) \(18 \mathrm{~g}\). of glucose per \(\mathrm{dm}^3\)
(c) 6 g. of urea in \(500 \mathrm{~cm}^3\)
(d) \(27 \mathrm{~g}\). of fructose per \(\mathrm{dm}^3\)
45. For isotonic solutions of non-electrolytes which one of the following is equal?
(a) Molality
(b) Normality
(c) Weight percent
(d) Molarity
46. A 0.1 \(\mathrm{M}\) aqueous solution of urea has osmotic pressure of 2 atm. A mixture is prepared by mixing
(i) \(50 \mathrm{ml}\) of \(0.2 \mathrm{M}\) glucose and
(ii) \(150 \mathrm{ml}\) of \(0.4 \mathrm{M}\) canesugar. Temperature being same, the osmotic pressure of the mixture would be
(a) \(7 \mathrm{~atm}\)
(b) \(8 \mathrm{~atm}\)
(c) \(12 \mathrm{~atm}\)
(d) \(6 \mathrm{~atm}\)
47. The mass of glucose to be dissolved in \(250 \mathrm{~cm}^3\) of water, to get a solution which is isotonic with 0.2 M solution of urea, is
(a) \(36 \mathrm{~g}\)
(b) \(9 \mathrm{~g}\)
(c) \(18 \mathrm{~g}\)
(d) \(4.5 \mathrm{~g}\)
48. In an aqueous solution the molecular mass of sodium chloride, as obtained from the colligative properties, is
(a) its normal molecular weight
(b) more than its normal molecular weight
(c) less than its normal molecular weight
(d) unpredictable
49. The boiling point of a decimolal aqueous solution of glucose would be
\(\left(\right.\) Given \(\mathrm{K}_{\mathrm{b}}=0.52 \mathrm{~K} \mathrm{~kg} \mathrm{~mol}^{-1}\) )
(a) \(100.52^{\circ} \mathrm{C}\)
(b) \(99.48^{\circ} \mathrm{C}\)
(c) \(99.948^{\circ} \mathrm{C}\)
(d) \(100.052^{\circ} \mathrm{C}\)
50. The example of solution of liquid in gas is
(a) dry air
(b) aerated water
(c) amalgam
(d) moist air

16. Chemical thermodynamics
1. Which of the following is not a state function?
(a) \(q_p\)
(b) \(q\)
(c) enthalpy
(d) entropy.
2. Which of the following is not an extensive property?
(a) molarity
(b) heat capacity
(c) mass
(d) volume.
3. In a chemical reaction work is done by the system when
(a) number of moles of gaseous reactants is equal to the number of moles of gaseous products
(b) total number of moles increases
(c) number of moles of gaseous substances decreases
(d) number of moles of gaseous products is greater than the number of moles of gaseous reactants.
4. The heat evolved in the following reaction \(2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{H}_2 \mathrm{O}(\mathrm{1}), \Delta \mathrm{H}=-484 \mathrm{~kJ}\) to produce \(9 \mathrm{~g}\) of water is
(a) \(484 \mathrm{~kJ}\)
(b) \(121 \times 10^3 \mathrm{~J}\)
(c) \(242 \mathrm{~kJ}\)
(d) \(968 \mathrm{~kJ}\)
5. When a sample of an ideal gas is allowed to expand at constant temperature against an atmospheric pressure,
(a) surroundings does work on the system
(b) \(\Delta \mathrm{U}=0\)
(c) no heat exchange takes place between the system and surroundings
(d) internal energy of the system increases.
6. In what reaction of the following, work is done by the system on the surroundings?
(a) \(\mathrm{Hg}(\mathrm{l}) \longrightarrow \mathrm{Hg}(\mathrm{g})\)
(b) \(3 \mathrm{O}_2\) (g) \(\longrightarrow 2 \mathrm{O}_3\) (g)
(c) \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{HCl}(\mathrm{g})\)
(d) \(\mathrm{N}_2(\mathrm{~g})+3 \mathrm{H}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{NH}_3(\mathrm{~g})\)
7. A gas does \(0.320 \mathrm{~kJ}\) of work on its surroundings and absorbs \(120 \mathrm{~J}\) of heat from the surroundings. Hence, \(\Delta U\) is
(a) \(440 \mathrm{~kJ}\)
(b) \(200 \mathrm{~J}\)
(c) \(120.32 \mathrm{~J}\)
(d) \(-200 \mathrm{~J}\)
8. In the reaction, \(\mathrm{H}_2(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{HCl}(\mathrm{g}) \Delta \mathrm{H}\) \(=-184 \mathrm{~kJ}\), if 2 moles of \(\mathrm{H}_2\) react with 2 moles of \(\mathrm{CI}_2\), then \(\Delta \mathrm{U}\) is equal to
(a) \(-184 \mathrm{~kJ}\)
(b) \(-368 \mathrm{~kJ}\)
(c) Zero
(d) \(+368 \mathrm{~kJ}\)
9. For which of the following substances \(\Delta_l H^{\circ}\) is not zero
(a) \(\mathrm{Ca}\) (s)
(b) \(\mathrm{He}(\mathrm{g})\)
(c) \(\mathrm{P}\) (red)
(d) \(\mathrm{CH}_3 \mathrm{OH}(1)\)
10. If for a reaction \(\Delta H\) is negative and \(\Delta S\) is positive then the reaction is
(a) spontaneous at all temperatures
(b) nonspontaneous at all temperatures
(c) spontaneous only at high temperatures
(d) spontaneous only at low temperatures
11. The relationship between \(\Delta G^6\) of a reaction and its equilibrium constant is
(a) \(-\Delta G^0=\frac{R T}{\ln K}\)
(b) \(\Delta \mathrm{G}^0=\frac{R T}{\ln \mathrm{K}}\)
(c) \(\frac{\mathrm{RT} \ln \mathrm{K}}{\Delta \mathrm{G}^0}=-1\)
(d) \(\Delta G^{\circ}=\) RT \(\ln \mathrm{K}\)
12. Which of the following has highest entropy?
(a) \(\mathrm{Al}(\mathrm{s})\)
(b) \(\mathrm{CaCO}_3\) (s)
(c) \(\mathrm{H}_2 \mathrm{O}\) (1)
(d) \(\mathrm{CO}_2(\mathrm{~g})\)
13. For the process, \(\mathrm{H}_2 \mathrm{O}(1) \longrightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g})\) at \(100^0 \mathrm{C}\), \(\Delta S\) is
(a) positive
(b) negative
(c) zero
(d) unpredictable
14. For the conversion of liquid to solid below the melting point of solid
(a) \(\Delta S_{\mathrm{ms}}\) is negative and the process is spontaneous
(b) \(\Delta \mathrm{S}_{\mathrm{m}}\) is positive and the process is spontaneous
(c) \(\Delta \mathrm{S}_{\mathrm{var}}\) is positive and the process is nonspontaneous
(d) \(\Delta S_{m s}\) is zero and the process is at equilibrium.
15. Which of the following conditions guarantee that a reaction is spontaneous at constant \(T\) and \(P\) ?
(a) entropy of system increases
(b) enthalpy of system decreases
(c) entropy of system decreases and that of surroundings increases
(d) Gibbs energy of the system decreases.
16. Which of the following processes is nonspontaneous?
(a) dissolving \(\mathrm{KCl}\) in water
(b) mixing of iodine vapour and nitrogen gas
(c) decomposition of \(\mathrm{NaCl}\) to \(\mathrm{Na}\) (s) and \(\mathrm{Cl}_2(\mathrm{~g})\)
(d) freezing of water at \(270 \mathrm{~K}\).
17. For which of the following' reactions \(\Delta S\) is negative?
(a) \(\mathrm{Mg}(\mathrm{s})+\mathrm{Cl}_2\) (g) \(\longrightarrow \mathrm{MgCl}_2\) (s)
(b) \(\mathrm{H}_2 \mathrm{O}(\mathrm{l}) \longrightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{g})\)
(c) \(\mathrm{CaCO}_3\) (s) \(\longrightarrow \mathrm{CaO}\) (s) \(+\mathrm{CO}_2\) (g)
(d) \(\mathrm{I}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{I}(\mathrm{g})\)
18. A gas expands in volume from \(2 \mathrm{~L}\) to \(5 \mathrm{~L}\) against a pressure of 1 atm at constant temperature. The work done by the gas will be
(a) \(3 \mathrm{~J}\)
(b) \(-303.9 \mathrm{~J}\)
(c) \(-303.9 \mathrm{~L}\).atm
(d) \(303.9 \mathrm{~L}\) atm
19. Given the reaction,

The enthalpy of formation of \(\mathrm{NH}_3\) is
(a) \(-92.6 \mathrm{~kJ}\)
(b) \(92.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
(c) \(-46.3 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
(d) \(-185.2 \mathrm{~kJ} \mathrm{~mol}^{-1}\)
20. Which of the following compounds will absorb maximum quantity of heat when dissolved in the same amount of water. The enthalpies of solution of these compounds at \(25^{\circ} \mathrm{C}\) in k.J \(/\) mole are given in brackets ?
(a) \(\mathrm{P}\left(\Delta \mathrm{H}=-32.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\right)\)
(b) \(\mathrm{Q}\left(\Delta \mathrm{H}=-17.3 \mathrm{~kJ} \mathrm{~mol}^{-1}\right)\)
(c) \(\mathrm{R}\left(\Delta \mathrm{H}=+2.56 \mathrm{~kJ} \mathrm{~mol}^{-1}\right)\)
(d) \(\mathrm{S}\left(\Delta \mathrm{H}=+25.6 \mathrm{~kJ} \mathrm{~mol}^{-1}\right)\)
21. For a reversible process at equilibrium, the change in entropy may be expressed as
(a) \(\Delta \mathrm{S}=\mathrm{Tq}_{\mathrm{rev}}\)
(b) \(\Delta \mathrm{S}=\frac{\mathrm{q}_{\mathrm{rev}}}{\mathrm{T}}\)
(c) \(\Delta S=\frac{\Delta H}{T}\)
(d) \(\triangle \mathrm{S}=\Delta \mathrm{G}\).
22. Free energy, may be defined as :
(a) \(\mathrm{G}=\mathrm{U}-\mathrm{TS}\)
(b) \(\mathrm{G}=\mathrm{U}+\mathrm{TS}\)
(c) \(\mathrm{G}=\mathrm{H}-\mathrm{TS}\)
(d) \(\mathrm{G}=\mathrm{U}+\mathrm{H}-\mathrm{TS}\).
23. When a solid changes into liquid, the entropy
(a) increases
(b) remains the same
(c) decreases
(d) becomes zero.
24. If \(q\) is the heat added to the system, \(W\) is the work done by the system and \(\triangle E\) is the change in internal energy, then according to first law of thermodynamics
(a) \(\Delta \mathrm{U}=\mathrm{q}+\mathrm{w}\)
(b) \(\Delta \mathrm{U}=\mathrm{q}-\mathrm{w}\)
(c) \(\Delta \mathrm{U}=\mathrm{q}+\mathrm{p} \Delta \mathrm{V}\)
(d) \(\Delta \mathrm{U}=\mathrm{q}+\Delta \mathrm{H}\)
25. Which of the following pairs has heat of neutralisation equal to \(-57.1 \mathrm{k} \mathbf{J}\) ?
(a) \(\mathrm{HNO}_3, \mathrm{KOH}\)
(b) \(\mathrm{HCl}, \mathrm{NH}_4 \mathrm{OH}\)
(c) \(\mathrm{H}_2 \mathrm{SO}_4, \mathrm{NH}_4 \mathrm{OH}\)
(d) \(\mathrm{CH}_3 \mathrm{COOH}, \mathrm{NaOH}\).
26. Free energy is related to enthalpy and entropy changes as
(a) \(\Delta \mathrm{G}=\Delta \mathrm{H}-\mathrm{T} \Delta \mathrm{S}\)
(b) \(\Delta \mathrm{G}=\mathrm{T} \Delta \mathrm{S}-\Delta \mathrm{H}\)
(c) \(\Delta \mathrm{G}=\frac{\Delta \mathrm{H}-\Delta \mathrm{S}}{\mathrm{T}}\)
(d) \(\Delta \mathrm{G}=\Delta \mathrm{H}+\mathrm{T} \Delta \mathrm{S}\)
27. A spontaneous change is one in which the system suffers
(a) Increase in internal energy
(b) Lowering of entropy
(c) Lowering of free energy
(d) No energy change
28. For which of the following reactions, \(\Delta S\) is not positive?
(a) \(\mathrm{I}_2(\mathrm{~s}) \rightarrow \mathrm{I}_2\) (g)
(b) \(\mathrm{CuO}(\mathrm{s})+\mathrm{H}_2\) (g) \(\rightarrow \mathrm{Cu}(\mathrm{s})+\mathrm{H}_2 \mathrm{O}(\ell)\)
(c) \(2 \mathrm{O}_3\) (g) \(\rightarrow 3 \mathrm{O}_2(\mathrm{~g})\)
(d) \(\mathrm{CH}_4\) (g) \(+2 \mathrm{O}_2\) (g) \(\rightarrow \mathrm{CO}_2\) (g) \(+\mathrm{H}_2 \mathrm{O}\) (g).
29. For an equilibrium state,
(a) \(\Delta \mathrm{H}>0\)
(b) \(\Delta \mathrm{G}>0\)
(c) \(\Delta \mathrm{H}=\mathrm{T} \Delta \mathrm{S}\)
(d) \(\Delta \mathrm{H}>\mathrm{T} \Delta \mathrm{S}\).
30. If there is no exchange of heat between the system and the surrounding during the process, it is called
(a) Isobaric process
(b) adiabatic process
(c) isothermal process
(d) irreversible process.
31. For the reaction, \(\mathrm{PCl}_5(\mathrm{~g}) \rightarrow \mathrm{PCl}_3(\mathrm{~g})+\mathrm{Cl}_2(\mathrm{~g})\)
(a) \(\Delta \mathrm{H}=\Delta \mathrm{U}\)
(b) \(\Delta \mathrm{H}>\Delta \mathrm{U}\)
(c) \(\Delta \mathrm{H}<\Delta \mathrm{U}\)
(d) None of the above.
32. An endothermic reaction is allowed to occur veryrapidly in the air. The temperature of the surrounding air
(a) remains constant
(b) decreases
(c) increases
(d) may increase or decrease.
33. Which of the following is not an intensive property ?
(a) Entropy
(b) Pressure
(c) Temperature
(d) Molar volume.
34. Which of the following is not a state function ?
(a) Heat
(b) Internal energy
(c) Enthalpy
(d) Entropy
35. For a chemical reaction at constant \(\mathbf{P}, \mathbf{\Delta H}\) is equal to
(a) zero
(b) \(\omega\)
(c) \(q / T\)
(d) \(\mathrm{zu}+\mathrm{p} \Delta \mathrm{V}\)
36. The enthalpy change for the process: \(\mathrm{C}(\mathrm{s}) \rightarrow \mathbf{C}(\mathrm{g})\) corresponds to enthalpy of
(a) fusion
(b) sublimation
(c) combustion
(d) vaporisation.
37. In an electrochemical cell, if \(\mathbf{E}\) is the e.m.f. of the cell involving \(\mathrm{n}\) mole of electrons, then \(\Delta G^0\) is
(a) \(\Delta \mathrm{G}^0=n \mathrm{nFE}^\theta\)
(b) \(\Delta \mathrm{G}^0=-\mathrm{nFE}\)
(c) \(\mathrm{E}^0=\mathrm{nF} \Delta \mathrm{G}^0\)
(d) \(\Delta \mathrm{G}^0=\frac{n F}{E^0}\)
38. For a spontaneous endothermic reaction:
(a) \(\Delta \mathrm{G}>0\)
(b) \(\Delta \mathrm{G}=0\)
(c) \(\Delta \mathrm{H}<0\)
(d) \(\Delta \mathrm{S}>\frac{\Delta \mathrm{H}}{\mathrm{T}}\)
39. For the reversible vaporisation of water at 373 \(\mathrm{K}\) and 1 atmospheric pressure, \(\Delta \mathrm{G}\) is equal to
(a) \(\Delta \mathrm{H}\)
(b) \(\triangle \mathrm{S}\)
(c) zero
(d) \(\Delta \mathrm{H} / \mathrm{T}\)
40. For a chemical reaction at constant \(P\) and \(V\), \(\Delta \mathrm{H}\) is equal to
(a) \(\Delta \mathrm{U}\)
(b) zero
(c) \(\Delta \mathrm{U}+\mathrm{P} \Delta \mathrm{V}\)
(d) \(\mathrm{p} / \mathrm{T}\)
41. \((\Delta \mathrm{U}-\Delta \mathrm{H})\) for the formation of \(\mathrm{NH}_3\) from \(\mathrm{N}_2\) and \(\mathrm{H}_2\) is
(a) \(-2^2 \mathrm{RT}\)
(b) \(2 \mathrm{RT}\)
(c) RT
(d) \(\frac{1}{2} \mathrm{RT}\)
42. The sign of \(\Delta \mathrm{G}\) for the process of melting of ice at \(260 \mathrm{~K}\) is
(a) \(\Delta \mathrm{G}=0\)
(b) \(\Delta \mathrm{G}<0\)
(c) \(\Delta \mathrm{G}>0\)
(d) None of these
43. A system absorbs \(\mathbf{1 0} \mathrm{k} . \mathrm{J}\) of heat at constant volume and its temperature rises from \(27^{\circ} \mathrm{C}\) to \(37^{\circ} \mathrm{C}\). The value of \(\Delta U\) is
(a) \(100 \mathrm{~kJ}\)
(b) \(10 \mathrm{~kJ}\)
(c) 0
(d) \(1 \mathrm{~kJ}\).
44. The enthalpies of combustion of carbon and carbon monoxide are \(-\mathbf{3 9 3 . 5} \&\) \& \(-\mathbf{2 8 3 . 0} \mathrm{k.J} \mathrm{mol}^{-}\) ' respectively. The enthalpy of formation of carbon monoxide is
(a) \(-676.5 \mathrm{~kJ}\)
(b) \(110.5 \mathrm{~kJ}\)
(c) \(-110.5 \mathrm{~kJ}\)
(d) \(676.5 \mathrm{~kJ}\).
45. The standard enthalpies of formation of \(\mathrm{HCl}(\mathrm{g})\), \(\mathrm{H}(\mathrm{g})\) and \(\mathrm{Cl}(\mathrm{g})\) are \(-92.2,217.7\) and \(121.4 \mathrm{~kJ}\) \(\mathrm{mol}^{-1}\) respectively. The bond dissociation energy of \(\mathrm{HCl}\) is
(a) \(+431.3 \mathrm{~kJ}\)
(b) \(236.9 \mathrm{~kJ}\)
(c) \(-431.3 \mathrm{~kJ}\)
(d) \(339.1 \mathrm{~kJ}\).
46. Calculate the heat required to make \(6.4 \mathrm{~kg}\) of \(\mathrm{CaC}_2\) from \(\mathrm{CaO}\) (s) and \(\mathrm{C}\) (s) from the reaction \(\mathrm{CaO}(\mathrm{s})+3 \mathrm{C}(\mathrm{s}) \rightarrow \mathrm{CaC}_2(\mathrm{~s})+\mathrm{CO}(\mathrm{g})\) given that \(\Delta_j \mathrm{H}^0(\mathrm{CaO})=-151.6 \mathrm{kcal}, \Delta_J \mathrm{H}^0\left(\mathrm{CaC}_2\right)=-14.2\) kcal, \(\Delta \mathrm{H}^{\bullet}(\mathrm{CO})=-26.4\) kcal.
(a) \(5624 \mathrm{kcal}\)
(b) \(1.1 \times 10^4 \mathrm{kcal}\)
(c) \(86.24 \times 10^3\)
(d) \(1100 \mathrm{kcal}\).
47. Four grams of helium is expanded from 1 atm to one-tenth of its original pressure at \(30^{\circ} \mathrm{C}\). Change in entropy (assuming ideal gas behaviour) is
(a) \(38.3 \mathrm{JK}^{-1}\)
(b) \(76.6 \mathrm{JK}^{-1}\)
(c) \(19.15 \mathrm{JK}^{-1}\)
(d) \(100 \mathrm{JK}^{-1}\)
48. 48. For the reaction at \(300 \mathrm{~K}\)
$$
\begin{aligned}
& A(\mathrm{~g})+\mathrm{E}(\mathrm{g}) \rightarrow \mathrm{C}(\mathrm{g}) \\
& \Delta \mathrm{U}=-3.0 \mathrm{kcal} \Delta S=-10.0 \mathrm{cal} / \mathrm{K} \\
& \left(\mathrm{R}=2 \mathrm{cal} \mathrm{mol}^{-1} \mathrm{~K}^{-1}\right)
\end{aligned}
$$
\(\Delta \mathrm{G}\) is
(a) \(-600 \mathrm{cal}\)
(b) \(-3600 \mathrm{cal}\)
(c) \(2400 \mathrm{cal}\)
(d) \(3000 \mathrm{cal}\).
49. For the reaction, \(2 \mathrm{H}_2 \mathrm{O}(\mathrm{g}) \rightarrow 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g})\) \(\Delta_1 \mathrm{H}^0\) of water is
(a) \(285.8 \mathrm{~kJ}\)
(b) \(-285.8 \mathrm{~kJ}\)
(c) \(1143.2 \mathrm{~kJ}\)
(d) \(1243.2 \mathrm{~kJ}\).
50. If equilibrium constant \(K\) is \(10^3\), the \(\Delta G^{\bullet}\) for the reaction at \(300 \mathrm{~K}\) is (assume \(R=8 \mathrm{JK}^{-1}\) \(\mathbf{m o l}^{-1}\) )
(a) \(-16.582 \mathrm{~kJ}\)
(b) \(16.582 \mathrm{~kJ}\)
(c) \(165.82 \mathrm{~kJ}\)
(d) \(1658.2 \mathrm{~kJ}\).

17. Electrochemistry
1. In the electrolysis of molten \(\mathrm{Al}_2 \mathrm{O}_3\) with inert electrodes
(a) \(\mathrm{Al}\) is axidized at anode to \(\mathrm{Al}^{\mathrm{l}}\)
(b) \(\mathrm{O}_2\) gas is produced at anode
(c) \(\mathrm{O}^{-}\)is reduced at cathode
(d) \(\mathrm{O}\) is oxidised at anode
2. The number of electrons that have a total charge of 965 coulo mbs is
(a) \(6.022 \times 10^{23}\)
(b) \(6.022 \times 10^{22}\)
(c) \(6.022 \times 10^4\)
(d) \(3.011 \times 10^{\mathrm{as}}\)
3. The time required to produce \(2 \mathrm{~F}\) of electricity with a current of 2.5 amperes is
(a) \(13.4 \mathrm{~h}\)
(b) 1200 min
(c) \(50000 \mathrm{~s}\)
(d) \(1.5 \mathrm{~h}\)
4. The number of Faradays required to produce 0.5 mol of free metal from \(\mathrm{Al}^{\mathrm{H}}\) is
(a) 3
(b) 2
(c) 6
(d) 1.5
5. The number of coulombs necessary to deposit 1 g of potassium metal ( molar mass \(39 \mathrm{e} \mathrm{mol}^{-1}\) ) from \(\mathrm{K}^+\) ions is
(a) \(96500 \mathrm{C}\)
(b) \(1.93 \times 10^3 \mathrm{C}\)
(c) \(1237 \mathrm{C}\)
(d) \(2474 \mathrm{C}\)
6. The strongest oxidizing agent among the species \(\ln ^{3+}\left(\mathrm{E}^2=-1.34 \mathrm{~V}\right), \mathrm{Au}^{3+}\left(\mathrm{E}^2=1.4 \mathrm{~V}\right), \mathrm{H}_{\mathrm{g}^2+}\left(\mathrm{E}^2=\right.\) \(0.85 \mathrm{~V}), \mathrm{Cr} \mathrm{r}^j(\mathrm{E}=-0.74 \mathrm{~V})\) is
(a) \(\mathrm{Cr}^2\)
(b) \(\mathrm{Au}=\)
(c) \(\mathrm{Hg}^{2+}\)
(d) \(\ln ^{3-}\)
7. The value of constant in Nernat equation \(\mathrm{E}=\mathrm{E}^0-\frac{\text { constant }}{\mathrm{n}}\) in \(\mathbf{Q}\) at \(25^{\circ} \mathrm{C}\) is
(a) \(0.0592 \mathrm{mV}\)
(b) \(0.0592 \mathrm{~V}\)
(c) \(25.7 \mathrm{mV}\)
(d) \(0.0296 \mathrm{~V}\)
8. The reaction, \(2 \mathrm{Br}^{-}(\mathrm{aq})+\mathrm{Sn}^{t+}(\mathrm{aq}) \Longrightarrow \mathrm{Br}_2(\mathrm{I})\) \(+\operatorname{Sn}(\bar{s})\) with the standard potentiak. \(\mathbf{E}^0 \mathrm{Sn}=-0.114 \mathrm{~V}, \mathbf{E}^0 \mathrm{_Br}_2=+1.09 \mathrm{~V}\), is
(a) spontaneous in reverse direction
(b) spontaneous in forward direction
(c) at equilibrium
(d) nonspontaneous in reverse direction.
9. Daniel cell operates under nonstandard state conditions. If the equation of the cell reaction is multiplied by 2 then
(a) \(E\) and \(E^{\prime}\) remain unchanged
(b) E is doubled
(c) \(n\) remains unchanged in Nernst equation.
(d) \(\mathrm{Q}\) is halved in Nernst equation.
10. Consider the cell, \(\mathrm{Pt}\left|\mathrm{H}_2(\mathrm{~g})\right| \mathbf{H}^{+}{ }_{(2 q)} \| \mathbf{I}(\mathrm{aq}) \mid\) \(I_2(\mathrm{~s})\). If the standard cell potential is \(0.54 \mathrm{~V}\) then the standard potential for cathode half reaction will be
(a) \(0 \mathrm{~V}\)
(b) \(-0.54 \mathrm{~V}\)
(c) \(+0.54 \mathrm{~V}\)
(d) \(1.08 \mathrm{~V}\)
11. Consider the cell \(\mathrm{Pt} \mid \mathrm{Cl}_2\) (g) \(\mid \mathrm{HCl}(\mathrm{aq}) \| \mathrm{HBr}\) (aq) \(\mid \mathrm{Br}_2\) (I) \(\mid \mathrm{Pt}\). If concentration of \(\mathrm{HCl}\) is increased, the cell potential will
(a) increase
(b) decrease
(c) remain the same
(d) become maximum
12. The standard potential for the cell reaction \(2 \mathrm{T1}(\mathrm{s})+\mathrm{Hg}^{2+}(1 \mathrm{M}) \longrightarrow 2 \mathrm{Ti}^{+}(1 \mathrm{M})+\mathrm{Hg}(\mathrm{l})\) where \(\mathrm{E}_{\mathrm{T} 1}^0=-0.34 \mathrm{~V}, \mathrm{E}_{\mathrm{H}_2}^0=0.86 \mathrm{~V}\) is
(a) \(0.52 \mathrm{~V}\)
(b) \(-0.52 \mathrm{~V}\)
(c) \(-1.2 \mathrm{~V}\)
(d) \(+1.2 \mathrm{~V}\)
13. \(\Delta \mathrm{G}^0\) for the reaction
\(\mathrm{Ag}^{+}(\mathrm{aq})+1 / \mathrm{H}_2(\mathrm{~g}) \longrightarrow \mathrm{H}^{+}(\mathrm{aq})+\mathrm{Ag}(\mathrm{s})\), where standard potential for silver half cell reaction is \(0.8 \mathrm{~V}\), will be
(a) \(-77.2 \mathrm{~kJ}\)
(b) \(+77.2 \mathrm{~kJ}\)
(c) \(154.4 \mathrm{~kJ}\)
(d) \(-38.6 \mathrm{~kJ}\)
14. The following reaction occurs in a galvanic cell
$$
2 \mathrm{Cu}^{+}+2 \mathrm{e}^{-} \longrightarrow \mathrm{Cu}
$$
If \(\mathrm{E}_{\mathrm{eu}^{+}}^0, \mathrm{Cua}^2+=+0.16 \mathrm{~V}\) and \(\mathrm{E}_{\mathrm{Cut} i \mathrm{ca}}^0=0.52 \mathrm{~V}\), the standard cell potential will be
(a) \(0.68 \mathrm{~V}\)
(b) \(0.36 \mathrm{~V}\)
(c) \(-0.36 \mathrm{~V}\)
(d) \(-0.68 \mathrm{~V}\)
15. The SI unit of molar conductivity is
(a) \(\mathrm{S} \mathrm{cm}^2 \mathrm{~mol}^{-1}\)
(b) \(\mathrm{S} \mathrm{dm}^2 \mathrm{~mol}^{-1}\)
(c) \(\mathrm{S} \mathrm{m}^2\)
(d) \(\mathrm{S} \mathrm{m}^2 \mathrm{~mol}^{-1}\)
16. Consider the following half reactions and choose the correct alternative
(i) \(\mathrm{Cl}_2\) (g) \(+2 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Cl}^{-}\)(aq) \(\mathrm{E}^0=1.36 \mathrm{~V}\)
(ii) \(\mathrm{Br}_2(\mathrm{I})+2 \mathrm{e}^{-} \longrightarrow 2 \mathrm{Br}^{-}\)(aq) \(\quad \mathrm{E}^0=1.07 \mathrm{~V}\)
(iii) \(\mathrm{I}_2(\mathrm{~s})+2 \mathrm{e}^{-} \longrightarrow 2 \mathrm{I}\)-(aq) \(\quad \mathrm{E}^0=0.53 \mathrm{~V}\)
(a) \(\mathrm{Br}_2\) cannot oxidize \(\mathrm{I}^{-}\)
(b) \(\mathrm{Cl}_2\) can oxidize \(\mathrm{Br}\) but not \(\mathrm{I}^{-}\)
(c) \(\mathrm{I}_2\) can oxidize \(\mathrm{Cl}^{-}\)
(d) \(\mathrm{Br}_2\) can oxidize \(\mathrm{I}^{-}\)but not \(\mathrm{Cl}^{-}\)
17. Maximum standard emf will be delivered by the cell consisting of the half cells
(a) \(\mathrm{F}^{-} \mid \mathrm{F}_2\) and \(\mathrm{Br} \mid \mathrm{Br}^2\)
(b) \(\mathrm{F}^{-} \mid \mathrm{F}_2\) and \(\mathrm{Li}^{+} \mid \mathrm{Li}\)
(c) \(\mathrm{Li}^{+} \mid \mathrm{Li}\) and \(\mathrm{Ca}^{2+} \mid \mathrm{Ca}\)
(d) \(\mathrm{Br} \mid \mathrm{Br}\), and \(\mathrm{Au}^{+} \mid \mathrm{Au}\).
18. During electrolysis, \(2 \mathrm{~A}\) current is passed through an electrolytic solution for \(965 \mathrm{~s}\). The number of moles of electrons passed will be
(a) 0.02
(b) 0.01
(c) 200
(d) 0.037
19. The same quantity of electricity is passed through two cells one containing \(\mathrm{AlCl}_3\) solution and the other containing \(\mathrm{ZnCl}_2\) solution. If 0.04 mole of \(\mathbf{A l}\) is produced in the first cell, the number of moles of \(\mathrm{Zn}\) produced in the second cell will be
(a) 0.08
(b) 0.0267
(c) 0.06
(d) 0.02
20. \(\mathbf{E}^0\) of an electrode half reaction is related to \(\Delta G^0\) by the equation, \(E^*=-\Delta G^0 / n F\). If the amount of \(\mathrm{Ag}^{+}\)in the half reaction
\(\mathbf{A g}^{+}+\mathrm{e}^{-} \longrightarrow \mathbf{A g}\) is tripled then
(a) \(\mathrm{n}\) is tripled
(b) \(\Delta G^{\circ}\) reduces to one third
(c) \(\mathrm{E}^0\) reduces to one third
(d) all the above
21. Electrolysis involves oxidation and reduction respectively at
(a) anode and cathode
(b) cathode and anode
(c) at both the electrodes
(d) none of these
22. When electricity is allowed to pass then which of the following is decomposed?
(a) Urea
(b) Glucose
(c) Ethyl alcohol
(d) \(\mathrm{AgNO}_3\)
23. The amount of ion discharged during electrolysis is not directly proportional to
(a) Resistance
(b) Time
(c) Current
(d) Chemical equivalent
24. During electrolysis the species discharged at cathode are
(a) ions
(b) cations
(c) anions
(d) all of these
25. Which of the following is not non-electrolyte?
(a) Acetic acid
(b) Glucose
(c) Ethanol
(d) Urea
26. The electric charge required to deposit one gram equivalent of an element is
(a) one ampere current
(b) 96500 coulombs per second
(c) charge on one mole of electrons
(d) both b and c
27. Which of the following is not a strong electrolyte?
(a) aqueous caustic potash
(b) acetic acid
(c) dil hydrochloric acid
(d) fused \(\mathrm{NaCI}\)
28. Which of the following is not a good conductor of electricity?
(a) \(\mathrm{NaCl}\) (Molten)
(b) \(\mathrm{NaCl}(\mathrm{aq})\)
(c) \(\mathrm{NaCl}(\mathrm{s})\)
(d) Silver metal
29. The number of Faradays required to produce \(0.5 \mathrm{~mol}\) of free metal from \(\mathrm{Al}^{3+}\) is
(a) 3
(b) 2
(c) 6
(d) 1.5
30. Prevention of corrosion of iron by Zn coating is called?
(a) Galvanization
(b) Cathodic protection
(c) Electrolysis
(d) Photoelectrolysis
31. In voltaic cell the two half cells are always connected by
(a) Salt bridge
(b) Porous partition
(c) Porous pot
(d) Any of these
32. The number of coulombs necessary to deposit \(\lg\) of potassium metal (molar mass \(39 \mathrm{~g} \mathrm{~mol}^{-1}\) ) from \(\mathrm{K}^{+}\)ions is
(a) \(96500 \mathrm{C}\)
(b) \(1.93 \times 10^5 \mathrm{C}\)
(c) \(1237 \mathrm{C}\)
(d) \(2474 \mathrm{C}\)
33. Which of the following metals can deposit copper from copper sulphate solution?
(a) Mercury
(b) Iron
(c) Gold
(d) Platinum
34. During working of Daniell cell
(a) Zn rod grows in size
(b) Cu rod grows in size
(c) \(\mathrm{Zn}\) rod reduces in size
(d) both \(\mathrm{b}\) and \(\mathrm{c}\)
35. For SHE arbitrarily
(a) \(\mathrm{Ps}_{\mathrm{S}}>\mathrm{P}_{\mathrm{O}}\)
(b) \(\mathrm{P}_{\mathrm{o}}>\mathrm{P}_{\mathrm{s}}\)
(c) \(\mathrm{P}_{\mathrm{s}}=\mathrm{P}_{\mathrm{O}}\)
(d) none of them
36. An electrode can be in a state of
(a) solid
(b) liquid
(c) gas
(d) all of these
37. When a gas like \(\mathrm{H}_2\) or \(\mathrm{Cl}_2\) are to be used as an electrode it is incorporated with
(a) The glass jacket
(b) Platinum as an inert electrode
(c) Al metal
(d) Another gas electrode
Pt The reduction reaction taking place at the cathode will be
(a) \(\frac{1}{2} \mathrm{Cl}_2+\mathrm{e}^{-} \rightarrow \mathrm{Cl}^{-}\)
(b) \(\frac{1}{2} \mathrm{O}_{2(y)} \rightarrow \frac{1}{2} \mathrm{O}_2^{2-}+2 \mathrm{e}^{-}\)
(c) \(2 \mathrm{OH}^{-} \rightarrow \frac{1}{2} \mathrm{O}_{2(g)}+\mathrm{H}_2 \mathrm{O}+\mathrm{e}^{-}\)
(d) \(\mathrm{Cl}_2 \rightarrow 2 \mathrm{Cl}^{-}+2 \mathrm{e}^{-}\)
39. The single electrode potential depends upon following factor
(a) temperature
(b) nature of electrode
(c) conc. of the electrolyte
(d) all of these
40. The redox reaction during discharging of the lead accumulator is
(a) \(\mathrm{Pb} \rightarrow \mathrm{Pb}^{2+}+2 \mathrm{e}^{-}\)
(b) \(\mathrm{PbO}_2+\mathrm{H}^{+}+\mathrm{SO}_2+2 \mathrm{e}^{-} \rightarrow \mathrm{PbSO}_4+2 \mathrm{H}_2 \mathrm{O}\)
(c) \(\mathrm{Pb}_{(01}+\mathrm{PbO}_{200}+2 \mathrm{H}_2 \mathrm{SO}_{44=0} \rightarrow 2 \mathrm{PbSO}_{400}+2 \mathrm{H}_2 \mathrm{O}\)
(d) \(2 \mathrm{PbSO}_{400}+2 \mathrm{H}_2 \mathrm{O} \rightarrow \mathrm{Pb}_{40}+\mathrm{PbO}_{200}+2 \mathrm{H}_2 \mathrm{SO}_{400}\)
41. Daniel cell operates under nonstandard state conditions. If the equation of the cell reaction is multiplied by 2 then
(a) \(E\) and \(E^u\) remain unchanged
(b) \(\mathrm{E}\) is doubled
(c) n remains unchanged in Nemst equation.
(d) \(Q\) is halved in Nernst equation.
42. Which of the following metal can not liberate \(\mathrm{H}_2\) gas from dilute hydrochloric acid?
(a) \(Z \mathrm{n}\)
(b) \(\mathrm{Mg}\)
(c) Al
(d) Ag
43. Calomel electrode is an example of
(a) Indicator electrode
(b) Primary reference electrode
(c) Secondary reference electrode
(d) None of these
44. Lead accumulator acts as
(a) voltaic cell
(b) electrolytic cell
(c) both of these
(d) none of these
45. Accurate measurement of the emf of an electrochemical cell is done by
(a) voltmeter
(b) potentiometer
(c) galvanometer
(d) conductometer
46. Which of the following metals is most readily corrodes in moist air?
(a) Al
(b) \(\mathrm{Fe}\)
(c) both 'a' and 'b'
(d) None of these
47. The time in hours required for a current of ampere to decompose electrolytically \(18 \mathrm{~g}\) of \(\mathrm{H}_2 \mathrm{O}\)
(a) 9
(b) 18
(c) 36
(d) 54
48. In air galvanic cell during its working if suddenly the salt bridge is removed then the potential of the cell
(a) Remains same
(b) Increases to maximum
(c) Decreases half the value
(d) Drops to zero
49. In a saturated calomel electrode the KCI solution is saturated with
(a) only \(\mathrm{KCl}\)
(b) only calomel
(c) both \(\mathrm{KCl}\) and calomel
(d) none
50. The ECE of a metal is " \(E\) " the amount of the metal deposited by the passage of \(500 \mathrm{~m} /\) current through the solution for 4 minutes will be
(a) \(2000 \mathrm{E} \mathrm{g} / \mathrm{F}\)
(b) \(1200 \mathrm{E} \mathrm{g} / \mathrm{F}\)
(c) \(120 \mathrm{E} \mathrm{g} / \mathrm{F}\)
(d) \(2 \mathrm{E} \mathrm{g} / \mathrm{F}\)
51. Which one of the following statements is correct?
(a) During the electrolytic dissociation, the number of cations and anions obtained are always equal
(b) The electrolytic dissociation leads reduction in the number of particles in the solution
(c) The cations and anions do not reunite to give back neutral molecules
(d) The number of positive and negative ions are equivalent
52. The equivalent weights of the elements \(A, \mathbf{B}\) and \(C\) are in inereasing order. Their weights deposited in electroly tic cells by the same quantity of electricity are \(x\), \(y\) and \(w\) respectively. Then
(a) \(x>y>w\)
(b) \(\mathrm{x}=\mathrm{Y}=\mathrm{w}\)
(c) \(x<y<w\)
(d) \(\mathrm{x}>\mathrm{Y} \leqslant \mathrm{w}\)
53. The main function of the salt bridge is
(a) to allow ions to go from one half cell to another
(b) to provide link between two half cells
(c) to keep the emf of the cell positive
(d) to maintain electrical neutrality of the solutions in the two half cells
54. The SI unit of molar conductivity is
(a) \(\mathrm{S} \mathrm{cm}^2 \mathrm{~mol}^{-1}\)
(b) \(\mathrm{S} \mathrm{dm}^2 \mathrm{~mol}^{-1}\)
(c) \(\mathrm{S} \mathrm{m}^2\)
(d) \(\mathrm{S} \mathrm{m}^2 \mathrm{~mol}^{-1}\)
55. Out of \(\mathrm{Cu}, \mathrm{Ag}, \mathrm{Fe}\) and \(\mathrm{Zn}\), the metal which can displace all others from their salt solutions is
(a) Ag
(b) \(\mathrm{Cu}\)
(c) \(\mathrm{Zn}\)
(d) Fe
56. Molar conductivity of a solution is \(1.26 \times 10^{-2}\) \(\mathbf{Q}^{-1} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\). It is molarity is 0.01. Its specific conductivity will be
(a) \(1.26 \times 10^{-15}\)
(b) \(1.26 \times 10^{-3}\)
(c) \(1.26 \times 10^{-4}\)
(d) 0.0063
57. Molar ionic conductivities of a bivalent electrolyte are 57 and 73 . The molar conductivity of the solution will be
(a) \(130 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
(b) \(65 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
(c) \(260 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
(d) \(187 \mathrm{~S} \mathrm{~cm}^2 \mathrm{~mol}^{-1}\)
58. A smuggler could not carry gold by chemically depositing iron on the gold surface since
(a) gold is denser
(b) iron rusts
(c) gold has higher reduction potential than iron
(d) gold has lower reduction potential than iron
59. On the basis of the information available for the reaction
$$
\begin{aligned}
& 4 / 3 \mathrm{Al}+\mathrm{O}_2 \rightarrow 2 / 3 \mathrm{Al}_2 \mathrm{O}_3 \\
& \Delta \mathrm{G}=-827 \mathrm{~kJ} / \mathrm{mol}
\end{aligned}
$$
of the minimum e.m.f. required to carry out electrolysis of \(\mathrm{Al}_2 \mathrm{O}_3\) is \(\left(\mathrm{F}=96500 \mathrm{C} \mathrm{mol}^{-1}\right)\)
(a) \(8.56 \mathrm{~V}\)
(b) \(2.14 \mathrm{~V}\)
(c) \(4.28 \mathrm{~V}\)
(d) \(6.42 \mathrm{~V}\)
60. Several blocks of magnesium are fixed to the bottom of a ship to
(a) keep away the sharks
(b) make the ship lighter
(c) prevent action of water and salt
(d) prevent puncturing by under-sea rocks.

18. Chemical Kinetics
1. The rate of a chemical reaction can be expressed in terms of
(a) rate of consumption of catalyst
(b) rate of consumption of reactants only
(c) rate of cousumption of reac tants and formation of products both
(d) rate of formation of products only.
2. The rate of a reaction is expressed in the units
(a) \(\mathrm{L} \mathrm{mol}^{-1} \mathrm{I}^{-1}\)
(b) mol \(\mathrm{dm}^{-1} \mathrm{t}^{-1}\)
(c) Ms
(d) \(\mathrm{M}^{-1} \mathrm{~s}^{-1}\)
3. In the reaction \(A+3 B \longrightarrow 2 C\), the rate of formation of \(\mathrm{C}\) is
(a) the same as rate of consumption of \(A\).
(b) the same as the rate of consumption of \(B\)
(c) twice the rate of consumption of \(\mathrm{A}\)
(d) \(3 / 2\) times the rate of consumption of \(\mathrm{B}\).
4. The instantaneous rate of reaction \(2 \mathrm{~A}+\mathrm{B}-\mathrm{C}\). \(+3 \mathrm{D}\) is given by
(a) \(\frac{\mathrm{dA}}{\mathrm{dt}}\)
(b) \(\frac{1}{2} d \mathrm{dt}\)
(c) \(\frac{\mathrm{d}[\mathrm{B}]}{\mathrm{dt}}\)
(d) \(\frac{1}{3} \frac{\mathrm{L}[\mathrm{D}]}{\mathrm{dt}}\)
5. A reaction is first order with respect to reactant \(A\) and second order with respect to reactant \(B\). The rate law for the reaction is given by
(a) rate \(=\mathrm{k}[\mathrm{A}][\mathrm{B}]^2\)
(b) rate \(=[\mathrm{A}][\mathrm{B}]^2\)
(c) rate \(=\mathrm{k}[\mathrm{A}]]^2[\mathrm{~B}]\)
(d) rate \(=\mathrm{k}[\mathrm{A}]^0[\mathrm{~B}]^2\)
6. Molecularity of an elementary reaction
(a) may be zero
(b) is always integgrai
(c) may be semi-integral
(d) may be integral, fractional or zero.
7. The unit of rate constant for first order reaction is
(a) min-2
(b) 5
(c) \(\mathrm{s}^{-11}\)
(d) \(\min\)
8. The integrated rate equation for first order reaction \(A \longrightarrow\) products is given by
(a) \(k=\frac{2.303}{1}\left[\mathrm{ln}]\mathrm{[A_0]}/[A_3]\right.\)
(b) \(k={ }_1^1[\mathrm{l}: \mathrm{A}]\)
(c) \(k=\frac{2.303}{t} \log _{10} \frac{[A]_2}{[A]}\) (d) \(k=\frac{1}{t} \ln \frac{[A]_0}{[A]}\)
9. The half life of a first order reaction is \(30 \mathrm{~min}\) and the initial concentration of the reactant is 0.1 M. If the initial concentration of reactant is doubled, then the half life of the reaction will be
(a) \(1800 \mathrm{~s}\)
(b) 60 min
(c) \(15 \mathrm{~min}\)
(d) \(900 \mathrm{~s}\)
10. The slope of the straight line obtained by plotting rate versus concentration of reactant for a first order reaction is
(a) \(-\mathrm{k}\)
(b) \(-\mathrm{k} / 2.303\)
(c) \(\mathrm{k} / 2.303\)
(d) \(\mathrm{k}\)
11. The reaction between \(\mathrm{H}_2(\mathrm{~g})\) and \(\mathrm{ICI}(\mathrm{g})\) occurs in the following steps:
i) \(\mathrm{H}_2+\mathrm{ICI} \longrightarrow \mathrm{HI}+\mathrm{HCl}\)
ii) \(\mathrm{HI}+\mathrm{ICI} \longrightarrow \mathrm{I}_2+\mathrm{HCI}\)
The reaction intermediate in the reaction is
(a) \(\mathrm{HCl}\)
(b) \(\mathrm{HI}\)
(c) 12
(d) ICl
12. Consider the reaction
\(2 \mathrm{NO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \longrightarrow 2 \mathrm{NO}_2(\mathrm{~g})\). If \(\frac{\mathrm{d}\left[\mathrm{NO}_2\right]}{\mathrm{dt}}=\)
\(0.052 \mathrm{M} / \mathrm{s}\) then \(\frac{\mathrm{d}\left[\mathrm{O}_2\right]}{\mathrm{dt}}\) will be
(a) \(0.052 \mathrm{M} / \mathrm{s}\)
(b) \(0.114 \mathrm{M} / \mathrm{s}\)
(c) \(0.026 \mathrm{M} / \mathrm{s}\)
(d) \(-0.026 \mathrm{M} / \mathrm{s}\)
13. The rate of the first order reaction
\(A \longrightarrow\) products is \(0.01 \mathrm{M} / \mathrm{s}\), when reactant concentration is \(0.2 \mathrm{M}\). The rate constant for the reaction will be
(a) \(0.05 \mathrm{~s}^{-1}\)
(b) 0.05 min \(^{-11}\)
(c) \(0.1 \mathrm{~s}^{-1}\)
(d) \(0.015^{-1}\)
14. The rate constant of a reaction
(a) decreases with increasing \(E\),
(b) decreases with decreasing \(E\),
(c) is independent of \(E\),
(d) decreases with increasing temperature
15. The slope of a graph \(\ln [A]_{\mathrm{E}}\) versus \(t\) for a first order reaction is \(-2.5 \times 10^{-2} \mathrm{~s}^{-1}\). The rate constant for the reaction \(w\) ill be
(a) \(5.76 \times 10^{-1} \mathrm{~s}^{-1}\)
(b) \(1.086 \times 10^{-3} \mathrm{~s}^{-1}\)
(c) \(-2.5 \times 10^{-1} \mathrm{~s}^{-1}\)
(d) \(2.5 \times 10^{-3} \mathrm{~s}^{-1}\)
16. Acatalyst increases the rate of the reaction by
(a) increasing \(E_a\)
(b) increasing \(\mathrm{T}\)
(c) decreasing \(E_{\text {. }}\)
(d) decreasing \(\mathrm{T}\)
17. The formation of \(\mathrm{SO}_3\) from \(\mathrm{SO}_2\) and \(\mathrm{O}_2\) takes place in the following steps
i) \(2 \mathrm{SO}_2+2 \mathrm{NO}_2 \longrightarrow 2 \mathrm{SO}_3+2 \mathrm{NO}\)
ii) \(2 \mathrm{NO}+\mathrm{O}_2 \longrightarrow 2 \mathrm{NO}_2\)
(a) \(\mathrm{NO}_2\) is intermediate
(b) \(\mathrm{NO}^2\) is catalyst
(c) \(\mathrm{NO}_2\) is catalyst and \(\mathrm{NO}\) is intermediate
(d) \(\mathrm{NO}_{\text {is catalyst and }} \mathrm{NO}\) is intermediate.
28. The reaction \(\left.\mathrm{H}_2 \mathrm{O}_{2(\mathrm{~b})} \rightarrow \mathrm{H}_2 \mathrm{O}_{(\mathrm{g})}+\mid \mathrm{O}\right]_{(2)}\) is a
(a) first order raction
(b) second order reaction
(c) zero order reaction
(d) third order reaction
29. Which equation is correct for first order reactions?
(a) \(t_{1 / 2} \propto C^{-1}\)
(b) \(\mathrm{t}_{1 / 2} \propto \mathrm{C}\)
(c) \(\mathrm{t}_{1 / 2} \propto \mathrm{C}^0\)
(d) \(\mathrm{t}_{1 / 2} \propto \mathrm{C}^{\mathrm{U} / 2}\)
30. In the sequence of raction
\(k_3>k_2>k_1\), then the rate determining step of the raction is
(a) \(\mathrm{A} \rightarrow \mathrm{B}\)
(b) \(\mathrm{B} \rightarrow \mathrm{C}\)
(c) \(\mathrm{C} \rightarrow \mathrm{D}\)
(d) \(\mathrm{A} \Rightarrow \mathrm{D}\)
31. For a Zero order raction, the plot of conc. of products vs time is linear with
(a) +ve slope and zero intercept
(b) -ve slope and zero intercept
(c) +ve slope and non-zero intercept
(d) -ve slope and non-zero intercept
32. A first order reaction has a half-life period of then the rate constant will be
(a) \(10^{-4} \mathrm{Sec}^{-1}\)
(b) \(10^{-3} \mathrm{Sec}^{-1}\)
(c) \(10^{-1} \mathrm{Sec}^{-1}\)
(d) \(6.93 \times 10^{-3} \mathrm{Sec}^{-1}\)
33. Ethyl acetate is hydrolysed in acidic medium. Its order of reaction and molecularity are respectively
(a) 1 and 1
(b) 1 and 2
(c) 2 and 1
(d) 2 and 2
34. Which of the following is a first order reaction?
(a) \(2 \mathrm{H}_2 \mathrm{O}_{2(\text { (aq) }} \stackrel{\mathrm{x}}{\longrightarrow} 2 \mathrm{H}_2 \mathrm{O}+\mathrm{O}_{2(\mathrm{~g})}\)
(b) \(2 \mathrm{HI} \rightarrow \mathrm{H}_2+\mathrm{I}_2\)
(c) \(2 \mathrm{NO}_2 \rightarrow 2 \mathrm{NO}+\mathrm{O}_2\)
(d) \(2 \mathrm{NO}+\mathrm{O}_2 \rightarrow 2 \mathrm{NO}_2\)
35. If a is the initial concentration and \(K\) is the rate constant of a zero reaction, the time for the reaction to go to completion will be
(a) \(\frac{k}{a}\)
(b) \(\frac{\mathrm{a}}{\mathrm{k}}\)
(c) \(\frac{a}{2 k}\)
(d) \(\frac{k}{2 a}\)
36. The activation energy for the forward reaction \(\mathrm{X} \rightarrow \mathrm{Y}\) is \(80 \mathrm{k} . \mathrm{J}\). The heat of reaction is \(200 \mathrm{k.J}\). The activation energy for the reaction \(B \rightarrow A\) will be
(a) \(80 \mathrm{~kJ}\)
(b) \(120 \mathrm{~kJ}\)
(c) \(280 \mathrm{~kJ}\)
(d) \(200 \mathrm{~kJ}\)
37. For a first order reaction, the ratio of times to complete \(99.9 \%\) and half of the raction is
(a) 2
(b) 3.323
(c) 6.656
(d) 10
38. On addition of \(\mathrm{AgNO}_3\) to \(\mathrm{NaCL}\). White ppt occurs
(a) Instantaneously
(b) with a measurable speed
(c) slowly
(d) depending on condition
39. The hydrolysis of ethyl acetate was carried out separately with \(0.005 \mathrm{M} \mathrm{HC}\) and \(0.05 \mathrm{M} \mathrm{H}_2 \mathrm{SO}_4\). The rate constants were found to be \(K_1\) and \(K_2\) respectively. Then
(a) \(\mathrm{k}_1=\mathrm{k}_2\)
(b) \(\mathrm{k}_1>\mathrm{k}_2\)
(c) \(\mathrm{k}_1<\mathrm{k}_2\)
(d) \(k_2=2 k_1\)
40.
The above plot is for order reaction to calculate value of rate constant.
(a) Second
(b) First
(c) Zero
(d) First and Zero
41. The half-life period for a first order reaction is 69.3 S. Its rate constantis
(a) \(10^{-2} \mathrm{~S}^{-1}\)
(b) \(10^{-4} \mathrm{~S}^{-1}\)
(c) \(10 \mathrm{~S}^{-1}\)
(d) \(10^2 \mathrm{~S}^{-1}\)
42. For a first order reaction we have \(k=100\) \(\operatorname{Sec}^{-1}\). The time for completion of \(50 \%\) reaction is
(a) 1 millisec
(b) 4 millisec
(c) 7 millisec
(d) 10 millisec
43. What is the half life of a radioactive substance if \(75 \%\) of its given amount disintegrate in 60 min?
(a) \(30 \mathrm{~min}\)
(b) \(45 \mathrm{~min}\)
(c) \(75 \mathrm{~min}\)
(d) \(90 \mathrm{~min}\)
44. The influence of temperature on reaction rate is predicted by
(a) Kirchoff's equation
(b) Arrhenious equation
(c) Van der wall's equation
(d) Kinetic equation
45. For the reaction \(A \rightarrow B\) when concentration of \(A\) is made 1.5 times the rate of reaction becomes 1.837 times. The order of reaction is
(a) 1
(b) 1.5
(c) 2
(d) 2.5
46. What is the order of a reaction which has arate expression rate \(=\mathbf{k} \mid \mathbf{A}]^{\mathbf{s i 2}}\left[\left.\mathrm{B}\right|^{-19}\right.\) ?
(a) \(\frac{3}{2}\)
(b) \(\frac{1}{2}\)
(c) Zero
(d) 1
47. The minimum energy needed to convert a reactant into product is called
(a) Potential energy
(b) Kinetic energy
(c) Threshold energy
(d) Activation energy
48. Threshold enerlgy in a reaction is equal to
(a) activation energy
(b) activation energy - normal energy
(c) activation energy + normal energy
(d) normal enerlgy of reactants only
49. The rate of a chemical reaction depends on
(a) Atomic Mass
(b) Equivalent Mass
(c) Molecular Mass
(d) Active Mass
50. For a General Chemical change \(2 \mathrm{~A}+3 \mathrm{~B} \rightarrow\) products, the rates with respect to \(A\) is \(r_1\) and with respect to \(B\) is \(r_2\). The rates \(r_1\) and \(r_2\) are related as
(a) \(3 r_1=2 r_2\)
(b) \(r_1=r_2\)
(c) \(2 r_1=3 r_2\)
(d) \(r_1{ }^2=2 r_2\)

19. General principle and process of isolation of elements
1. The ores that are concentrated by flotation method are
(a) Carbonates
(b) Sulphides
(c) Oxides
(d) Phosphates
2. In blast furnace, iron oxide is reduced by
(a) Silica
(b) \(\mathrm{CO}\)
(c) \(\mathrm{C}\)
(d) Lime stone
3. Which of the following metal is obtained by leaching its ore with dilute cyanide solution
(a) Silver
(b) Titanium
(c) Vanadium
(d) Zinc
4. Van Arkel method of purification of metals involves converting the metal to a
(a) Volatile compound
(b) Volatile unstable compound
(c) Non-volatile stable compound
(d) Non-volatile unstable compound
5. Zone refining is a method to obtain
(a) Very high temperature
(b) Ultra pure AI
(c) Ultra pure Germanium
(d) Ultra pure oxides
6. Electrochemical process (electrolysis of fused salt) is used to extract
(a) Iron
(b) Lead
(c) Sodium
(d) Silver
7. In metallurgy, flux is a substance used to convert
(a) Mineral into silicate
(b) Fusible impurities to infusible impurities
(c) Infusible impurities to soluble impurities
(d) Soluble impurities into infusible impurities
8. \(\mathrm{Fe}_2 \mathrm{O}_3\) is reduced to spongy iron near the top of blast furnace by
(a) \(\mathrm{CO}\)
(b) \(\mathrm{CO}_2\)
(c) \(\mathrm{C}\)
(d) \(\mathrm{H}_2\)
9. Highest carhon content iron is
(a) Stainless steel
(b) Wrought iron
(c) Cast iron
(d) Mild iron
10. During smelting silica is added to roasted copper ore to remove
(a) Cuprous Sulphide
(b) Cuprous Oxide
(c) Ferrous oxide
(d) Ferrous sulphide
11. The ore which contains copper and iron both
(a) Malachite
(b) Chalcopyrite
(c) Chalcocite
(d) Azurite
12. In extraction of iron limestone is used for
(a) Formation of slag
(b) Reduction of Fe Ore
(c) Purification of Fe formed
(d) Oxidation of Fe Ore
13. The process of extracting the metal from its ore is called
(a) Refining
(b) Concentration
(c) Leaching
(d) Metallurgy
14. Roasting results in the production of metal in the case of
(a) iron pyrites
(b) galena
(c) cinnabar
(d) bauxite
15. The method for the purification of impure metals which is based upon the phenomenon of electrolysis is called
(a) Electrorefining
(b) Hydrometallurgy
(c) Poling
(d) Liquation
16. Van Arkel method is used for refining
(a) lead
(b) zirconium
(c) vanadium
(d) zinc
17. The metal always found in free state is
(a) Gold
(b) Silver
(c) Copper
(d) Sodium
18. In blast furnace, iron oxide is reduced by
(a) \(\mathrm{SiO}_2\)
(b) \(\mathrm{CO}\)
(c) dil. \(\mathrm{HCl}\)
(d) limestone
19. Parke's process is used in the extraction of
(a) \(\mathrm{Zn}\)
(b) Fe
(c) Ag
(d) \(\mathrm{Na}\)
20. In electrorefining, the impure the metal is made
(a) cathode
(b) anode
(c) may be cathode or anode
(d) none of these
21. The iron used in the froth flotation method for the purification of ores is
(a) Coconut oil
(b) Kerosene oil
(c) Mustard oil
(d) Pine oil
22. Roasting is generally done in case of
(a) Oxide ores
(b) Silicate ores
(c) Sulphide ores
(d) Carbonate ores
23. The following equation depicts a method of purification of nickel by \(\mathrm{Ni}+4 \mathrm{CO} \stackrel{320 \mathrm{~K}}{\longrightarrow} \mathrm{NI}(\mathrm{CO})_4 \stackrel{420 \mathrm{~K}}{\longrightarrow} \mathrm{NICO}_4\)
(a) Zone refining
(b) Mond's method
(c) Van Arkel method (d) Cupellation
24. In metallurgy, flux is a substance used to convert
(a) insoluble impurities to a fusible mass.
(b) minerals into silicates
(c) soluble particles into insoluble particles
(d) fusible impurities to infusible impurities.
25. Which of the following is generally found in free state?
(a) \(\mathrm{Cu}\)
(b) \(\mathrm{Al}\)
(c) \(\mathrm{Au}\)
(d) \(\mathrm{Fe}\)
26. In aluminothermic process aluminium acts as
(a) Oxidising agent
(b) reducing agent
(c) flux
(d) none of these
27. Which of the following ores cannot be concentrated by froth floatation process?
(a) Bauxite
(b) Cinnabar
(b) Galena
(d) Copper pyrite.
28. Gold is usually-found near the mineral:
(a) Galena
(b) Quartz
(c) Mica
(d) Dolomite
29. Hoop's process is used for the purification of
(a) \(\mathrm{Al}\)
(b) \(\mathrm{Pb}\)
(c) \(\mathrm{Ag}\)
(d) \(\mathrm{Zn}\)
30. Horn silver is :
(a) \(\mathrm{Ag}_2 \mathrm{~S}\)
(b) \(\mathrm{AgCl}\)
(c) \(\mathrm{Ag}_2 \mathrm{~S} \cdot \mathrm{Sb}_2 \mathrm{~S}_3\)
(d) Pure silver.
31. Which of the following is not an ore of aluminium?
(a) Feldspar
(b) Corundum
(c) Bauxite
(d) Carnallite.
32. Which of the following is not present in type metal?
(a) \(\mathrm{Pb}\)
(b) \(\mathrm{Cu}\)
(c) \(\mathrm{Sn}\)
(d) \(\mathrm{Sb}\)
33. Which of the following alloys does not contain copper?
(a) Delta metal
(b) German silver
(c) Alnico
(d) Phosphorus bronze
34. The metal essential for plant life is
(a) Zinc
(b) Iron
(c) Nitrogen
(d) Potassium.
35. In electrorefining of copper, pure copper is obtained
(a) at cathode
(b) at anode
(c) in the electrolyte
(d) in anode mud
36. The ore of copper is
(a) Malachite
(b) Apatite
(c) Dolomite
(d) Haematite.
37. Calcination is carried out in a
(a) Blast furnace
(b) Electric furnace
(c) Bessemer convertor
(d) Reverberatory furnace.
38. A metal obtained by hydrometallurgy is
(a) \(\mathrm{Ag}\)
(b) \(\mathrm{Fe}\)
(c) \(\mathrm{Na}\)
(d) \(\mathrm{Mg}\)
39. The mineral of magnesium is
(a) Bauxite
(b) Malachite
(c) Carnallite
(d) Haematite.
40. Iron is extracted from magnetite by reduction with
(a) \(\mathrm{C}\)
(b) \(\mathrm{Mg}\)
(c) \(\mathrm{AI}\)
(d) \(\mathrm{H}_2\)
41. Iron obtained from blast furnace is known as
(a) wrought iron
(b) cast iron
(c) pig iron
(d) steel
42. Copper is refined by
(a) Smelting
(b) Roasting
(c) Electrolytic method
(d) Heating in a blast furnace
43. Pyrargyrite is
(a) \(\mathrm{Ag}_2 \mathrm{~S}\)
(c) \(\mathrm{AgCl}\)
(d) \(\mathrm{Ag}_2 \mathrm{~S}\)
44. An example of halide ores is
(a) Galena
(b) Bauxite
(c) Cryolite
(d) Cinnabar,
45. Carbon reduction method is used in the extraction of
(a) \(\mathrm{Sn}\)
(d) \(7 \mathrm{n}\)
(c) \(\mathrm{Pb}\)
(d) All
46. High purity copper metal is obtained by
(a) Carbon reduction
(b) Hydrogen reduction
(c) Electrolytic reduction
(d) Thermite reduction
47. Silica is added to roasted copper ore during smelting in order to remove
(a) cuprous oxide
(b) cuprous sulphide
(c) ferrous sulphide
(d) ferrous oxide
48. Which of the following ore is best concentrated by froth flotation process?
(a) magnetite
(b) cassiterite
(c) galena
(d) malachite
49. During the process of electrolytic refining of copper some metals present as impurity settle as "anode mud". These are
(a) Sn and \(\mathrm{Ag}\)
(b) \(\mathrm{Pb}\) and \(\mathrm{Zn}\)
(c) \(\mathrm{Ag}\) and \(\mathrm{Au}\)
(d) \(\mathrm{Fe}\) and \(\mathrm{Ni}\)
50. Heating \(\mathrm{Cu}_2 \mathrm{O}\) and \(\mathrm{Cu}_2 \mathrm{~S}\) will give
(a) \(\mathrm{Cu}+\mathrm{SO}_2^2\)
(b) \(\mathrm{Cu}+\mathrm{SO}_3\)

20. P BLOCK ELEMENTS
1. \(\mathrm{PCl}_5\) exists but \(\mathrm{NCl}_5\) does not due to
(a) Inertness of \(\mathrm{N}_2\)
(b) \(\mathrm{NCl}_5\) is unstable
(c) Larger size of \(\mathrm{N}\)
(d) Non-availability of vacant d-atomic orbitals
2. The p-p-p angle in white phosphorous is
(a) \(120^{\circ}\)
(b) \(109^{\circ} 28^{\prime}\)
(c) \(90^{\circ}\)
(d) \(60^{\circ}\)
3. Catalytic oxidation of \(\mathrm{NH}_3\) gives
(a) Nitric oxide
(b) Nitrogen
(c) Nitrogen dioxide
(d) dinitrogen pentoxide
4. Oxygen molecule shows
(a) Para magnetism
(b) Diamagnetism
(c) Ferro magnetism
(d) fern magnetism
5. When \(\mathrm{SO}_2\) is passed through acidified \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) solution.
(a) The solution turns green
(b) The solution is decolourized
(c) Reduction of \(\mathrm{SO}_2\) takes place
(d) Green ppt of \(\left.\mathrm({Cr}_2, \mathrm{SO}_4\right)_3\), is formed
6. \(\mathrm{FeSO}_4\) forms brown ring with
(a) \(\mathrm{NO}_3\)
(b) \(\mathrm{NO}_2\)
(c) \(\mathrm{NO}\)
(d) \(\mathrm{N}_2 \mathrm{O}_3\)
7. The shape of IF molecules is
(a) Tetrahedral
(b) Octahedral
(c) trigonal bipyramidal
(d) Pentagon bipyramidal
8. The strongest oxidizing agent is
(a) \(\mathrm{HOCl}\)
(b) \(\mathrm{HClO}_4\)
(c) \(\mathrm{HClO}_3\)
(d) \(\mathrm{HClO}_2^{-}\)
9. Iodine can exist in the oxidation states
(a) \(+1,+3,+5\)
(b) \(-1,+1,+3\)
(c) \(+3,+5,+7\)
(d) \(-1,+1,+3,+5,+7\)
10. Fluorine does not show positive oxidation state due to absence of
(a) d-orbitals
(b) s-orbitals
(c) p-orbitals
(d) f-orbitals
11. Structure of \(\mathrm{NH}_3\) is
(a) Trigonal
(b) Tetrahedral
(c) Pyramidal
(d) Trigonal bipyramidal
12. The most stable allotropic form of Sulphur is
(a) Rhombic Sulphur (b) Flowers of Sulphur
(c) Plastic Sulphur
(d) Mono clinic Sulphur
13. The basicity of phosphorous acid \(\left(\mathrm{H}_3 \mathrm{PO}_3\right)\) is
(a) one
(b) two
(c) four
(d) three
14. Which of the following halogen is radioactive in. nature?
(a) Chlorine
(b) Bromine
(c) Iodine
(d) Astatine
15. \(\mathrm{Cl}_2\) is liberated from the cold hydrochloric acid by the action of
(a) \({\mathrm{KMnO}_4}\)
(b) \(\mathrm{K}_2 \mathrm{MnO}_4\)
(c)\(\mathrm{K}_2\mathrm{Cr}_2\mathrm{O}_7\)
(d) \(\mathrm{K}_2 \mathrm{CrO}_4\)
17. Maximum covalency of nitrogen is
(a) two
(b) four
(c) three
(d) five
18. General electronic configuration of group 16 elements is
(a) \(n^2 \mathrm{np}^3\)
(b) \(\mathrm{ns}^2-\mathrm{np}^4\)
(c) \(n s^2 n p^2\)
(d) \(\mathrm{ns}^2 \mathrm{np}^5\)
19. \(\mathrm{NH}_3\) is basic while \(\mathrm{PH}_3\) is
(a) acidic
(b) neutral
(c) amphoteric
(d) basic
20. Name the noble gas not present in the air
(a) Radon
(b) Argon
(c) Krypton
(d) Helium
21. Which is the most abundant noble gas?
(c) \(\frac{\text { Argon }}{\text { Neon }}\)
(b) Helium
(d) Krypton
22. Noble gas used in the miner's cap lamp is
(a) krypton
(b) argon
(c) helium
(d) radon
23. A mixture used for respiration by the sea divers is
(a) \(\mathrm{He}+\mathrm{O}_2\)
(b) \(\mathrm{Ne}+\mathrm{O}_2\)
(c) \({\mathrm{Ar}+\mathrm{O}_2}\)
(d) \(\mathrm{Kr}+\mathrm{O}_2\)
24. Hybridization involved in \(\mathrm{H}_2 \mathrm{O}\) is
(a) \(\mathrm{sp}\)
(b) \(\mathrm{sp}^{3}\)
(c) \(\mathrm{sp}^3 \mathrm{~d}^2\)
(d) \(\mathrm{sp}^2\)
25. The structure of \(\mathrm{SF}_4\) is
\(\begin{array}{ll}\text { (a) Octahedral } & \text { (b) Bipyramidal }\end{array}\)
(c) Square planner
(d) Tetrahedral
26. Which one has the highest bond energy?
(a) \(\mathrm{O}-\mathrm{O}\)
(b) \(\underline{S-S}\)
(c) Se-Se
(d) \(\mathrm{Te}-\mathrm{Te}\)
27. Ozone is
(a) A compound of oxygen
(b) An allotropic oxygen
(c) An isotope of oxygen
(d) An isobar of oxygen
28. Which one of the following does not exist?
(a) \({\mathrm{HeF}^4}\)
(b) \(\mathrm{XeF}_4\)
(c)\(\mathrm{CF}_4\)
(d) \(\mathrm{SF}_6\)
29. Most powerful oxidizing agent is
(a) Fluorine
(b) Chlorine
(c) Iodine
(d) Bromine
30. Which one has lowest boiling point?
(a) \(\mathrm{H}_2 \mathrm{O}\)
(b) \(\underline{H}_2 \underline{\mathrm{S}}\)
(c) \(\mathrm{H}_2 \mathrm{Se}\)
(d) \(\mathrm{H}_2 \mathrm{Te}\)
31. Maximum covalency of Sulphur is
(a) Four
(b) \(\underline{\mathrm{Six}}\)
(c) Three
(d) Two
31. Which one is the strongest reducing agent?
(a) \(\mathrm{HF}\)
(b) \(\mathrm{HCl}\)
(c) \(\mathrm{HBr}\)
(d) \(\mathrm{HI}\)
32. Oxygen exhibit-1 oxidation state in
(a) \(\mathrm{OF}_2\)
(b) \({\mathrm{H}_2} \mathrm{O}_2\) (c)HCIO (d)\(\mathrm{H}_2 {O}\)
33. Fluorine can exist in the oxidation state.
(a) -1 only
(b) -1 and +1
(c) \(-1,+1,+3\) only
(d) \(-1,+1,+3,+7\)
34. Among the oxide \(\mathrm{M}_2 \mathrm{O}_3\), teh highest acidic nature is when, \(M\) is
(a) As
(c) \(\mathrm{Bi}\)
(b) \(\frac{\mathrm{P}}{\mathrm{Sb}}\)
35. Phosphorescence is exhibit by \(\begin{array}{ll}\text { (a) Yellow phosphorus} & \text { (b) Red phosphorus} \end{array}\)
(c) Both These
(d) None of these
36. The oxidation state of \(\mathrm{O}\)-atom is +2 in
(a) \(\mathrm {O}\mathrm{~F}_2\))
(b) \(\mathrm{O}_2 \mathrm{~F}_2\)
(c)\(\mathrm{H}_2\mathrm{O}\)
(d) \(\mathrm{H}_2 \mathrm{O}_2\)
37. Ozone is readily soluble in
(a) Turpentine oil
(b) Glacial acetic acid
(c) Water
(d) Both ' \(a\) ' and ' \(b\) '
38. The peroxy linkage is present in
(a) Marshali's acid
(b) Sulphuric acid
(c) Oleum
(d) None of these
39. Nitrogen (I) oxide is obtained by
(a) heating \(\mathrm{NH}_4, \mathrm{NO}_3\)
(b) heating \(\mathrm{NH}_4 \mathrm{NO}_2\)
(c) disproportionation of \(\mathrm{N}_2 \mathrm{O}_4\)
(d) the action of \(\mathrm{Cu}\) with conc. \(\mathrm{HNO}_3\)
40. Strong \(p^\pi, p^\pi\) bonds given by
(a) \(\mathrm{P}\)
(b) \(\mathrm{N}\)
(c) \(\mathrm{Bi}\)
(d) As
41. A gaseous mixture contains \(\mathrm{O}_2\) and \(\mathrm{N}_2\) is ratio of \(1: 2\) by mass. The ratio of their number of molecules is
(a) \(1: 2\)
(b) \(2: 1\)
(c) \(4: 7\)
(d) \(7: 16\)
42. The group-16 elements are usually known as
(a) Rare earths
(b) Pseudo-halogens
(c) coinage elements
(d) chalcogens
43. Caro's acid is
(a) \(\mathrm{H}_2 \mathrm{SO}_4\)
(b) \(\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_8\)
(c) \(\mathrm{H}_2 \mathrm{~S}_2 \mathrm{O}_7\)
(d) \(\mathrm{H}_2 \mathrm{SO}_3\)
44. A solution of \(\mathrm{Cl}_2\) in water contains
(a) \(\mathrm{HOCl}\) only
(b) \(\mathrm{HCl}\) only
(c) \(\mathrm{HOCl}\) and \(\mathrm{HCl}\) only
(d) \(\mathrm{HCl}, \mathrm{HOCl}\), and \(\mathrm{Cl}_2\)
45. Among the following, the most basic in character is
(a) \(\mathrm{Br}^{-}\)
(b) \(\underline{\mathrm{F}}=\)
(c) \(\mathrm{Cl}^{-}\)
(d) \(\mathrm{I}^{-}\)
46. The correct order of thermal stability of halogen acids is
(a) \(\mathrm{HI}>\mathrm{HBr}>\mathrm{HCl}>\mathrm{HF}\)
(b) \(\mathrm{HCl}>\mathrm{HF}>\mathrm{HBr}>\mathrm{HI}\)
(c) \(\mathrm{HF}>\mathrm{HCl}>\mathrm{HBr}>\mathrm{HI}\)
(d) \(\mathrm{HF}>\mathrm{HCl}>\mathrm{HI}>\mathrm{HBr}\)
47. In xenon tetrafluoride, the hybridised state of Xe, is
(a) \(\mathrm{sp}^3\)
(b) \(\mathrm{sp}^3 \mathrm{~d}^3\)
(c) \(\mathrm{dsp}^2\)
(d) \(\underline{s p}^3 \mathrm{~d}^2\)
48. Which one of following has zero valency?
(a) \(\mathrm{Si}\)
(b) \(\mathrm{Sc}\)
(c) \(\mathrm{Ca}\)
(d) Ar
49. The number of lone pair(s) of electrons present on \(\mathrm{Xe}\)-atom in \(\mathrm{XeOF}_4\) is/are
(a) 2
(b) \({1}\)
(c) 3
(d) 0
50. The gas, used for inflating aero plane Tyres, is
(a) \(\mathrm{H}_2\)
(b) \(\mathrm{He}\)
(c) \(\mathrm{N}_2\)
(d) \(\mathrm{Ar}\)

21. D AND F BLOCK ELEMENTS
1. The most common oxidation state of lanthanoids is
(a) +4
(b) +3
(c) +6
(d) +2
2. Which of the following elements belong to actinoid series?
(a) Cerium
(b) Lutetium
(c) Thorium
(d) Lanthanum
3. Which element among the lanthanoids has the smallest atomic radius?
(a) Cerium
(b) Lutetium
(c) Europium
(d) Gadolinium
4. The properties of \(\mathrm{Zr}\) and \(\mathrm{Hf}\) are similar because
(a) both have same atomic radii
(b) both belong to d block
(c) both belong to same series
(d) both have same number of electrons
5. Lanthanoids are placed in
(a) \(3^{\text {rd }}\) group and \(7^{\text {th }}\) period
(b) \(3^{\text {nd }}\) group and \(6^{\text {th }}\) period
(c) \(4^{\text {th }}\) group and \(7^{\text {th }}\) period
(d) \(3^{\text {rd }}\) group and \(5^{\text {th }}\) period
6. The general electronic configuration \([R n] 5 f^{1-14} 6 d^{0-1} 7 s^2\) is of the
(a) Lanthanoids
(b) Actinoids
(c) s-block elements
(d) d-block elements
7. The general electronic configuration of transition element is
(a) \((\mathrm{n}-1) \mathrm{d}^{1-10}\)
(b) (n-1) \(\mathrm{d}^{10} \mathrm{~ns}^2\)
(c) \((\mathrm{n}-1)^{1-10} \mathrm{~ns}^{1-2}\)
(d) (n-1) \(d^5 n s^1\)
8. An atom with atomic number 21 belongs to category of
(a) S-block elements
(b) P-block elements
(c) d-block elements
(d) f-block elements
9. The first transition element is
(a) Chromium
(b) Scandium
(c) Nickel
(d) Copper
10. Which one of the following has largest radii?
(a) \(\mathrm{Cr}^{3+}\)
(b) \(\mathrm{Mn}^{3+}\)
(c) \(\mathrm{Fe}^{3+}\)
(d) \(\mathrm{Co}^{3+}\)
11. Chromium ( \(Z=24)\) has clectronic configuration
(a) \([\mathrm{Ar}] 4 \mathrm{~d}^4 4 \mathrm{~s}^2\)
(b) \([\mathrm{Ar}] 4 \mathrm{~d}^5 4 \mathrm{~s}^1\)
(c) \([\mathrm{Ar}] 3 \mathrm{~d}^5 3 \mathrm{~s}^1\)
(d) \([\mathrm{Ar}] 3 \mathrm{~d}^5 4 \mathrm{~s}^1\)
12. The number of delectrons retained in \(\mathrm{Fe}^{2+}\) \((\mathrm{Z}\) of \(\mathrm{Fe}=26)\) ion is
(a) 3
(b) 4
(c) 5
(d) 6
13. Manganese achieves the highest oxidation state in its compounds
(a) \(\mathrm{Mn}_3 \mathrm{O}_4\)
(b) \(\mathrm{KMnO}_4\)
(c) \(\mathrm{K}_2 \mathrm{MnO}_4\)
(d) \(\mathrm{MnO}_2\)
14. Which one of the following element forms interstitial compounds?
(a) Fe
(b) \(\mathrm{Co}\)
(c) \(\mathrm{Ni}\)
(d) \(\mathrm{Sc}\)
15. Equivalent mass of \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) in acidic solution is
(a) molecular mass/2
(b) molecular mass/4
(c) molecular mass/l
(d) molecular mass/6
16. The group which belongs to transition series is
(a) 2
(b) 7
(c) 13
(d) 15
17. Which of the following compounds is expected to be coloured?
(a) \(\mathrm{AgNO}_3\)
(b) \(\mathrm{CuSO}_4\)
(c) \(\mathrm{ZnCl}_2\)
(d) \(\mathrm{CuCl}\)
18. Chromyl chloride test is for
(a) Chloride salt
(b) nitrate salt
(c) thiosulphate salt .
(d) sulphate salt
19. The product of 0 xidation of \(\mathrm{I}^{-}\)with \(\mathrm{MnO}_4^{-}\)ions in alkaline medium is
(a) \(\mathrm{I}_2\)
(b) \(\mathrm{IO}^{-}\)
(c) \(\mathrm{IO}_4^{-}\)
(d) \(\mathrm{IO}_3^{-}\)
20. The metal ion which is NOT coloured, is
(a) \(\mathrm{Fe}^3\)
(b) \(\mathrm{V}^2\)
(c) \(7 n^{2+}\)
(d) \(\mathrm{Ti}^{3+}\)
21. A gas when passed through the \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) and dil. \(\mathrm{H}_2 \mathrm{SO}_4\) solution; turns it green, the gas is
(a) \(\mathrm{H}_2 \mathrm{~S}\)
(b) \(\mathrm{NH}_3\)
(c) \(\mathrm{Cl}_2\)
(d) \(\mathrm{SO}_2\)
22. Transition metals and their oxides are used in processes of industries as
(a) Insecticides
(b) medicines
(c) detergents
(d) catalysts
23. Acidified potassium dichromate can NOT oxidize
(a) Ethanol
(b) Potassium iodide
(c) Ferric sulphate
(d) Hydrogen sulphide
24. The number of moles of \(\mathrm{KMnO}_4\) that will be required to react with one mole of sulphide ion in acidic medium is
(a) \(2 / 5\)
(b) \(3 / 5\)
(c) \(4 / 5\)
(d) \(5 / 5\)
25. The species with an atom in +6 oxidation state is
(a) \(\mathrm{MnO}_4^{-}\)
(b) \(\operatorname{Cr}(\mathrm{CN})_6^{3-}\)
(c) \(\mathrm{NiF}_6^{2-}\)
(d) \(\mathrm{CrO}_2 \mathrm{Cl}_2\)
26. If carbon is added to iron to prepare interstitial compound then the iron becomes
(a) Less tensile
(b) softer
(c) Less malleable
(d) more ductile
27. When conc. \(\mathrm{H}_2 \mathrm{SO}_4\) is added to \(\mathrm{KMnO}_4\) explosion occurs. The compound formed is
(a) \(\mathrm{MnSO}_4\)
(b) \(\mathrm{MnO}_5\)
(c) \(\mathrm{Mn}_2 \mathrm{O}_3\)
(d) \(\mathrm{Mn}_2 \mathrm{O}^3\)
28. Highest magnetic moment is shown by the ion
(a) \(\mathrm{V}^{3+}\)
(b) \(\mathrm{Co}^{3+}\)
(c) \(\mathrm{Fe}^{3+}\)
(d) \(\mathrm{Cr}^{3+}\)
29. Transition elements show negative oxidation states only in
(a) Complexes
(b) sulphides
(c) halides
(d) sulphates
30. The equation
\( $$
3 \mathrm{MnO}_4^{-}+4 \mathrm{H}^{+} \rightarrow 2 \mathrm{MnO}_4^{-}+\mathrm{MnO}_2+2 \mathrm{H}_2 \mathrm{O}
$$ \)
represents
(a) reduction
(b) disproportionation
(c) oxidation in acidic medium
(d) reduction in acidic medium.
31. Magnetic moment of \(\mathrm{Ce}^{3+}\) ion on the basis of 'spin-only' formula will be B.M.
(a) 1.232
(b) \(\overline{1} .332\)
(c) 1.532
(d) 1.732
32. Highest \((+7)\) oxidation state is shown by
(a) \(\mathrm{Co}\)
(b) Cr
(c) V
(d) \(\mathrm{Mn}\)
33. Which is heaviest among the following.
(a) Iron
(b) Copper
(c) Gold
(d) Silver
34. Which of the following compounds is not coloured?
(a) \(\mathrm{Na}_2\left[\mathrm{CuCl}_4\right]\)
(b) \(\mathrm{Na}_2\left[\mathrm{CdCl}_4\right]\)
(c) \(\mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]\)
(d) \(\mathrm{K}_3\left(\mathrm{Fe}\left(\mathrm{CN}_6\right)\right]\)
35. The element with an atomic number 26 is
(a) A non-metal
(b) Krypton
(c) Iron
(d) Manganese
36. \(\mathrm{Fe}^{3+}\) compounds are more stable than \(\mathrm{Fe}^{2+}\) compounds because
(a) \(\mathrm{Fe}^{3+}\) has smaller size than \(\mathrm{Fe}^{2+}\)
(b) \(\mathrm{Fe}^{3+}\) has \(3 \mathrm{~d}^5\) configuration (half-filled)
(c) \(\mathrm{Fe}^{3+}\) has higher oxidation state
(d) \(\mathrm{Fe}^{3+}\) is paramagnetic in nature
37. What happens when potassium iodide reacts with acidic solution of potassium dichromate?
(a) It liberates iodine.
(b) Potassium sulphate is formed.
(c) Chromium sulphate is formed.
(d) All the above products are formed.
38. Transition metals make the most efficient catalysts because of their ability to
(a) adopt multiple oxidation states and to form complexes
(b) form coloured ions
(c) show paramagnetism due to unpaired electrons
(d) form a large number of oxides.
39. The salts of \(\mathrm{Cu}\) in +1 oxidation state are unstable because
(a) \(\mathrm{Cu}^{+}\)has \(3 \mathrm{~d}^{10}\) configuration
(b) \(\mathrm{Cu}^{+}\)disproportionates easily to \(\mathrm{Cu}(\mathrm{O})\) and \(\mathrm{Cu}^{2+}\)
(c) \(\mathrm{Cu}^{+}\)disproportionates easily to \(\mathrm{Cu}^{2+}\) and \(\mathrm{Cu}^{3+}\)
(d) \(\mathrm{Cu}^{+}\)is easily reduced to \(\mathrm{Cu}^{2+}\).
40. The second and third row elements of transition metals resemble each other much more than they resemble the first row because of
(a) Lanthanoid contraction which results in almost same radii of second and third row metals
(b) diagonal relationship between second and third row elements
(c) similar ionisation enthalpy of second and third row elements
(d) similar oxidation states of second and third row metals.
41. Which of the following is not correctly matched with the given example?
(a) An element of first transition series which has highest second ionisation enthalpy-Cu
(b) An element of first transition series with highest third ionisation enthalpy- \(Z \mathrm{n}\)
(c) An element of first transition series with lowest enthalpy of atomisation- Zn
(d) Last element of third transition series-Cd
42. Which of the following statements concerning lanthanide elements is false?
(a) All lanthanides are highly dense metals.
(b) More characteristic oxidation state of lanthanide elements is +3 .
(c) Lanthanides are separated from one another by ion exchange method.
(d) Ionic radii of trivalent lanthanides steadily increases with increase in the atomic number.
43. Transitional elements are named transition elements because their characters are
(a) In between \(s\) and p-block elements
(b) Like that of \(p\) and \(d\)-block elements
(c) They are members of I.A. group
(d) They are like inactive elements
44. Colour of transition metal ions are due to absorption of some wavelength. This results in
(a) d-s transition
(b) s-s transition
(c) s-dtransition
(d) d-d transition.
45. Which of the following statements is not correct?
(a) Copper liberates hydrogen from acids.
(b) In higher oxidation states, manganese forms stable compounds with oxygen and fluorine.
(c) \(\mathrm{Mn}^ {3+}\) and \(\mathrm{CO}^{3+}\) are oxidising agents in aqueous solution.
(d) \(\mathrm{Ti}^{2+}\) and \(\mathrm{Cr}^{2+}\) are reducing agents in aqueous solution.
46. Which of the following statements is not true in regard to transition elements.
(a) They readily form complex compounds
(b) They show variable valency
(c) All their ions are colourless
(d) Their ions contain partially filled d-elelctron levels
47. Potassium dichromate is prepared from
(a) chromate obtained by the fusion of chromite ore
with sodium carbonate in free access of air
(b) pyrolusite which is fused with potassium hydroxide in the presence of air
(c) iron pyrite by the fusion with potassium carbonate in presence of moisture
(d) none of these.
48. A transition element \(\mathrm{X}\) has a configuration \(\left[\mathrm{Ar} \mid 3 \mathrm{~d}^4\right.\) in its +3 oxidation state. Its atomic number is
(a) 25
(b) 26
(c) 22
(d) 19
49. What is the total number of inner transition elements in the periodic table?
(a) 10
(b) 14
(c) 30
(d) 28
50. Transition metals are related to which block.
(a) s-block
(b) p-block
(c) d-block
(d) None of these

22. CO-ORDINATION COMPOUNDS
1. Which of the following statement is CORRECT? (CFSE = Crystal Field Splitting Energy)
a) Lower CFSE favours formation of low spin complex
b) Higher CFSE favours formation of high spin complex
c) A particular metal ion in a particular oxidation state can form either diamagnetic complexes only or paramagnetic complexes only.
d) \(t_{28}\) orbitals are three-fold degenerate while \(e_B\) orbitals are two-fold degenerate
2. An example of ambidentate ligand is
a) ammine
b) chloro
c) oxalato
d) thiocyanate
3. Which of the following is a complex compound?
a) \(\mathrm{KCl}, \mathrm{MgCl}_2, 6 \mathrm{H}_2 \mathrm{O}\)
b) \(\mathrm{K}_2 \mathrm{SO}_4 \cdot \mathrm{Al}_4\left(\mathrm{SO}_4\right)_3 \cdot 24 \mathrm{H}_2 \mathrm{O}\)
c) \(\left[\mathrm{Co}(\mathrm{ONO})\left(\mathrm{NH}_3\right)_5\right] \mathrm{SO}_4\)
d) \(\mathrm{VO}_2 \mathrm{Cl}_2\)
4. Ligands in a complex salt are
a) anions linked by coordinate bonds to a central metal atom or ion
b) cations linked by coordinate bonds to a central metal atom or ion
c) only molecules linked by coordinate bonds to a central metal atom or ion
d) ions or molecules linked by coordinate bonds to a central atom or ion
5. The coordination number of copper in cuprammonium sulphate is
a) 2
b) 3
c) 4
d) 6
6. In the complexes \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\) and \(\left[\mathrm{Cr}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}\) , the respective coordination number of iron and chromium are
a) 6 and 3
b) 3 and 3
c) 6 and 6
d) 3 and 6
7. IUPAC name of \(\mathrm{K}_4\left[\mathrm{Fe}(\mathrm{CN})_6\right]\) is
a) tetrapotassium ferrocyanide
b) potassium ferricyanide
c) potassium ferrocyanide
d) potassium hexacyanoferrate
8. IUPAC name of \(\mathrm{K}_3\left[\mathrm{Fe}(\mathrm{CN})_6\right]\) is
a) potassiumferrocyanide
b) potassiurnferricyanide
c) potassiurnhexacyanoferrate (II)
d) potassiurnhexacyanoferrate (III)
9. IUPAC name of \(\mathrm{K}_3\left[\mathrm{Al}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]\) is
a) potassiumalumino-oxalato
b) potassiumaluminium(III)trioxalate
c) potassiumtrioxalatoaluminate(III)
d) potassiumtrisoxalatoaluminate(III)
10. In the coordination compound, \(\mathrm{K}_4\left[\mathrm{Ni}(\mathrm{CN})_4\right]\), the oxidation state of nickel is
a) -1
b) 0
c) +1
d) +2
11. Among the following ions which one has the highest paramagnetism ?
a) \(\left[\mathrm{Cr}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
b) \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
c) \(\left[\mathrm{Cu}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
d) \(\left[\mathrm{Zn}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{2+}\)
12. Which one of the following is expected to be a paramagnetic complex ?
a) \(\left[\mathrm{Ni}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
b) \(\left[\mathrm{Ni}(\mathrm{CO})_4\right]\)
c) \(\left[\mathrm{Zn}\left(\mathrm{NH}_3\right)_4\right]^{2+}\)
d) \(\left[\mathrm{Sc}\left(\mathrm{H}_2 \mathrm{O}\right)\right]^{3+}\)
13. The compound which does NOT show paramagnetism is
a) \(\left[\mathrm{Cu}\left(\mathrm{NH}_3\right)_4\right] \mathrm{Cl}_2\)
b) \(\left[\mathrm{Ag}\left(\mathrm{NH}_3\right)_2\right] \mathrm{Cl}\)
c) \(\left[\mathrm{FeF}_6\right]^{3-}\)
d) \(\left[\mathrm{T}\left(\mathrm{H}_2 \mathrm{O}\right)_6\right]^{3+}\)
14. Ethylenediamine is an example of
a) monodentate ligand
b) bidentate ligand
c) tridentate ligand
d) polydentate ligand
15. The colour of hexaamminecobalt(III) chloride is
a) yellow orange
b) red
c) violet
d) green
16. A group of atoms can function as a ligand only when
a) it is a small molecule
b) it has an unshared electron pair
c) it is a negatively charged ion
d) it is a positively charged ion
17. Which of the following ligand is the most strong field ligand?
a) \(\mathrm{CO}\)
b) \(\mathrm{H}_2 \mathrm{O}\)
c) \(\mathrm{OH}^{-}\)
d) \(\mathrm{Ox}^{2-}\)
18. Among the following the most stable complex is
a) \(\left[\mathrm{Fe}\left(\mathrm{H}_2 \mathrm{O}\right)_4\right]^{3+}\)
b) \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\)
c) \(\left[\mathrm{FeI}_6\right]^{3-}\)
d) \(\left[\mathrm{FeCl}_6\right]^{3-}\)
19. \(\mathrm{CN}\) - is a strong field ligand. This is due to the fact that
a) it is a pseudo halide
b) it is a strong base
c) it forms high spin complex with metal species
d) it carries negative charge.
20. The coordination number of a central metal in a complex is determined by
a) the number of ligands around a metal ion bonded by sigma and pi bonds
b) the number of ligands around a metal ion bonded by only pi-bonds
c) the number of ligands around a metal ion bonded by only sigma bonds
d) the number of only anionic ligands bonded to the metal ion
21. Which one of the following complexes is outer orbital complex?
a) \(\left[\mathrm{Co}\left(\mathrm{C}_2 \mathrm{O}_4\right)_3\right]^{3-}\)
b) \(\left[\mathrm{Mn}(\mathrm{CN})_6\right]^{3-}\)
c) \(\left[\mathrm{Fe}(\mathrm{CN})_6\right]^{3-}\)
d) \(\left[\mathrm{CoF}_6\right]^{3-}\)
22. The oxidation state of \(\mathrm{Cr}\) in \(\left[\mathrm{Cr}\left(\mathrm{NH}_3\right)_4 \mathrm{Cl}_2\right]^{+}\)is
a) +3
b) +2
c) +1
d) 0
23. Which one of the following is a tridentate ligand?
a) trien
b) oxalato ion
c) EDTA
d) dien
24. Which of the following represents a chelating ligand?
a) \(\mathrm{H}_2 \mathrm{O}\)
b) \(\mathrm{NH}_3\)
c) DMG
d) \(\mathrm{Cl}^{-}\)
25. An ambidentate ligand is one which ?
a) is linked to the metal atom at two points
b) has two donor atoms but only one of them has the capacity to form a coordinate bond
c) has two donor atoms but either of the two can form a co-ordinate bond
d) forms chelate rings
26. A ligand having unshared pair of electron can be
a) a group containing a lone pair of electrons
b) a neutral molecule
c) a negatively charge ion
d) all of the above
27. A monodentate ligand has _.
a) one co-ordination site
b) two co-ordination sites
c) any number of co-ordination sites
d) no capacity to co-ordinate
28. \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{NO}_2\right] \mathrm{SO}_4\) and \(\left[\mathrm{Co}\left(\mathrm{NH}_3\right)_5 \mathrm{SO}_4\right] \mathrm{NO}_2\) exhibit isomerism.
a) co-ordination
b) linkage
c) ionization
d) optical
29. Which one of the following statements is INCORRECT?
a) Greater the stability constant of a complex ion, greater is its stability.
b) Greater the charge on the central metal ion, greater is the stability of the complex.
c) Greater is the basic character of the ligand, the greater is the stability of the complex.
d) Chelate complexes have low stability constants.
30. Which of the following is NOT optically active?
a) \(\left[\mathrm{Co}(\mathrm{en})_3\right]^{3+}\)
b) \(\left[\operatorname{Cr}(o x)_3\right]^{3-}\)
c)cis- \(\left[\mathrm{CoCl}_2(\mathrm{en})_2\right]^{+}\)
d) trans- \([\mathrm{CoCl} 12 (\mathrm {en})_2]^{+}\)
31. Which of the following statements is INCORRECT?
a) Co-ordination compounds and complexes are synonymous terms.
b) Complexes always give ions in the solution.
c) Complexes may give ions in the solution or may not give ions in the solution.
d) Complex ion does not dissociate into its component parts even in the solution.
32. In \(\mathrm{Ni}(\mathrm{CO})_4\), each carbonyl ligand donate electron pair(s) to \(\mathrm{Ni}\).
a) 1
b) 2
c) 3
d) 4
33. The value of \(x\) which appears in the complex \(\left[\mathrm{Ni}(\mathrm{CN})_4\right] \mathrm{x}\) is
a) +2
b) -2
c) 0
d) 4
34. What is the shape of \(\mathrm{Fe}(\mathrm{CO}) \mathrm{s}\) ?
a) Square pyramidal
b) Octahedral
c) Linear
d) Trigonal bipyramidal
35. The number of unpaired electrons in low spin octahedral complexes of \(\mathrm{Cr}^{2+}\left(\mathrm{d}^4\right)\) and \(\mathrm{Fe}^{3+}\left(\mathrm{d}^5\right)\) are respectively.
a) 0 and 1
b) 2 and 1
c) 0 and 2
d) 1 and 0
36. Coordination isomerism is caused by the interchange of ligands between the
a) cis and trans structure
b) complex cation and complex anion
c) inner sphere and outer sphere
d) low oxidation and higher oxidation states
37. The neutral ligand is
a) chloride
b) hydroxide
c) hydroxyl amine
d) hydrazinium
38. A strong ligand gives a complex which is generally called
a) high spin
b) high energy
c) low spin
d) stable
39. The complex ions \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_5,\left(\mathrm{NO}_2\right)\right]^{2+}\) and \(\left[\mathrm{Co}\left(\mathrm{H}_2 \mathrm{O}\right)_5-(\mathrm{ONO})\right]^{2+}\) are called. [Mar 2013]
a) linkage isomers
b) ionization isomers
c) co-ordination isomers
d) geometrical isomers
40. Which of the following is a common donor atom in ligands?
a) Arsenic
b) Nitrogen
c) Oxygen
d) Both b) and c)
41. Which one of the following octahedral complexes.will NOT show geometrical isomerism ( \(a\) and \(b\) are monodentate ligands)?
a) \(\left[\mathrm{Ma}_5 \mathrm{~b}\right]\)
b) \(\left[\mathrm{Ma}_2 \mathrm{~b}_4\right]\)
c) \(\left[\mathrm{Ma}_3 \mathrm{~b}_3\right]\)
d) \(\left[\mathrm{Ma}_4 \mathrm{~b}_2\right]\)
42. According to Werner's theory
a) primary valency can be ionized
b) secondary valency can be ionized
c) primary and secondary valencies both cannot be ionized
d) only primary valency cannot be ionized
43. The oxidation number of cobalt in \(\mathrm{K}\left[\mathrm{Co}(\mathrm{CO})_4\right]\) is (NCERT)
a) +1
b) +3
c) -1
d) -3

23. Halogens derivatives of alkanes and arenes
1. Halofonns are trihalogen derivatives of
a) \(\mathrm{C}_2 \mathrm{H}_6\)
b) \(\mathrm{CH}_4\)
c) \(\mathrm{C}_3 \mathrm{H}_8\)
d) \(\mathrm{C}_2 \mathrm{H}_4\)
2. In haloalkanes, halogen atom is bonded to hybridized carbon atom of an alkyl group.
a) \(\mathrm{sp}\)
b) \(s p^2\)
c) \(\mathrm{sp}^3\)
d) \(\operatorname{sp}^3 d\)
3. Primary, secondary and tertiary alkyl halides are derivatives of alkanes.
a) monohalogen
b) dihalogen
c) trihalogen
d) tetrahalogen
4. Which of the following is known as Freon?
a) \(\mathrm{CCl}_2 \mathrm{~F}_2\)
b) \(\mathrm{CHCl}_3\)
c) \(\mathrm{CH}_2 \mathrm{~F}_2\)
d) \(\mathrm{CHF}_3\)
5. Number of secondary alkyl halides possible for \(\mathrm{C}_3 \mathrm{H}_{11} \mathrm{Cl}\) are
a) 2
b) 3
c) 4
d) 5
6. Chloroform is slowly oxidised by air in the presence of light to form
a) chloropicrin
b) phosgene
c) dichloromethane
d) freon
7. Which of the following reactions is NOT an electrophilic substitution reaction of haloarenes?
a) Friedel-Craft's reaction
b) Nitration
c) Halogenation
d) Wurtz-Fittig reaction
8. The IUPAC name of chloroform is
a) chloromethane
b) halomethane
c) trichloromethane
d) chlorotrimethane
9. The IUPAC name of the given compound is

a) 2-Methyl-1-chlorobutane
b) 1-Chloro-2,2-dimethylpropane
c) 2,2-Dimethyl-1-chloropentane
d) 2,2-Dimethyl-3-chloropropane
10. The IUPAC name of isobutyl chloride is
a) 1-chloro-2-methylbutane
b) 1-chloro-2-methylpropane
c) 2-chlorobutane
d) 2-chloro-2-methylpropane
11. \(\mathrm{CH}_3-\mathrm{CH}-\mathrm{CHCl}-\mathrm{CH}_3\) is
a) 3-chloro-2-ethylbutane
b) 2-chloro-3-ethylpentane
c) 2-chloro-3-ethylbutane
d) 2-chloro-3-methylpentane
12. How many different isomers are possible for the molecular formula \(\mathrm{C}_3 \mathrm{H}_6 \mathrm{Cl}_2\) ?
a) 8
b) 6
c) 4
d) 2
13. Nitrating mixture is
a) conc. \(\mathrm{HNO}_3+\) conc. \(\mathrm{H}_2 \mathrm{SO}_4\)
b) conc. \(\mathrm{HNO}_3+\) conc. \(\mathrm{HCI}\)
c) conc. HCI + conc. \(\mathrm{H}_2 \mathrm{SO}_4\)
d) conc. \(\mathrm{HNO}_3+\) conc. \(\mathrm{NaOH}\)
14. The molecular formula of DDT has _ chlorine atoms.
a) 5
b) 4
c) 3
d) 2
15. In the energy profile diagram of \(\mathrm{S}_{\mathrm{N}}{ }^2\) mechanism, difference in energies of products and reactants is denoted as
a) \(\Delta \mathrm{H}\)
b) \(\Delta \mathrm{E}\)
c) \(E_3\)
d) \(\Delta \mathrm{U}\)
16. In \(\mathrm{S}_{\mathrm{N}}^2\) mechanism, the rate of reaction is proportional to concentration of
a) only substrate
b) only reagent
c) both substrate and reagent
d) neither substrate nor reagent
17. The order of reactivities of the following alkyl halides for \(\mathrm{S}_{\mathrm{N}}^2\) reaction is
a) \(\mathrm{RF}>\mathrm{RCI}>\mathrm{RB}_{\mathrm{r}}>\mathrm{RI}\)
b) \(\mathrm{RF}>\mathrm{RBr}>\mathrm{RCI}>\mathrm{RI}\)
c) \(\mathrm{RCI}>\mathrm{RBr}>\mathrm{RF}>\mathrm{RI}\)
d) \(\mathrm{RI}>\mathrm{RBr}>\mathrm{RCI}>\mathrm{RF}\)
18. Depletion of ozone layer is caused by
a) freons
b) alkanes
c) Grignard reagents d) carboxylic acids
19. What is \(\mathrm{A}\) and \(\mathrm{B}\) in given reaction ?

20. Molecular formula of dichloromethane is
a) \(\mathrm{CHCl}_3\)
b) \(\mathrm{CCl}_4\)
c) \(\mathrm{CH}_2 \mathrm{Cl}_2\)
d) \(\mathrm{CH}_3 \mathrm{Cl}\)
21. Which of the following reactions are most common in alkyl halides?
a) Nucleophilic substitution
b) Nucleophilic addition
c) Electrophilic addition
d) Electrophilic substitution
22. Wurtz synthesis is carried out by treating
a) alkyl halide with anhydrous soudim hydroxide in dry ether
b) alkyl halide with sodalime in dry ether
c) alkyl halide with sodium metal in dry ether
d) alkyl halide in aqueous solution of sodium hydroxide
23. The reaction of phosphorus tribromide with ethanol gives_derivative of alkanes.
a) monohalogen
b) dihalogen
c) trihalogen
d) polyhalogen
24. The \(\mathrm{C}-\mathrm{X}\) bond cleavage in aryl halide is more difficult than the \(\mathrm{C}-\mathrm{X}\) bond cleavage in alkyl halide due to
a) electromeric effect
b) positive inductive effect
c) negative inductive effect
d) resonance effect
25. \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Cl}+\mathrm{KCN}\) (aq.) \(\longrightarrow \mathrm{X}+\mathrm{Y}\). Compounds \(\mathrm{X}\) and \(\mathrm{Y}\) are respectively.
a) \(\mathrm{C}_6 \mathrm{H}_6, \mathrm{KCl}\)
b) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CN}, \mathrm{KCl}\)
c) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_3, \mathrm{KCl}\)
d) None of these
26. Why is chloroform stored into dark coloured bottles?
a) To prevent it from evaporating.
b) To protect it from moisture.
c) To prevent it from oxidation to form phosgene.
d) To prevent its reaction with glass.
27. Dipole moment of chlorobenzene is
a) more than cyclohexyl chloride
b) lower than cyclohexyl chloride
c) equal to cyclohexyl chloride
d) none ofthese
28. If two halogen atoms are attached to same carbon atom of an alkane, it is called as
a) alkyl halide
b) alkylene halide
c) alkylidenehalide d) aryl halide
29. The general molecular formula of monohalogen derivatives of alkanes is
a) \(\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}} \mathrm{X}_2\)
b) \(\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}}+{ }_1 \mathrm{X}\)
c) \(\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}-1} \mathrm{X}_3\)
d) \(\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}} \mathrm{X}\)
30. Isopropyl halide is a alkyl halide.
a) primary
b) secondary
c) tertiary
d) all of these
31. IUPAC name of \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}-\mathrm{CH}_2-\mathrm{CHI}-\mathrm{CH}_3\) is
a) 2-Iodo-4,4-dimethylbutane
b) 4-Iodo-2,2-dimethylpentane
c) 3-Iodo-4,4-dimethylpentane
d) 2-Iodo-4,4-dimethylpentane
32. In direct iodination of an alkane, the reverse reaction due to ill is prevented by using
a) mercuric oxide
b) iodic acid
c) dil. nitric acid
d) all of these
33. \(\mathrm{A} \mathrm{S}_{\mathrm{N}}^2\) reaction takes place with
a) retention of configuration
b) inversion of configuration
c) racemisation
d) all of these
34. Lucas reagent is an equimolar mixture of
a) concentrated \(\mathrm{HCI}+\) anhydrous \(\mathrm{ZnCl}_2\)
b) dilute \(\mathrm{HCI}+\) hydrated \(\mathrm{ZnCl}_2\)
c) concentrated \(\mathrm{HNO}_3+\) anhydrous zeci,
d) concentrated \(\mathrm{HCI}+\) anhydrous \(\mathrm{MgCl}_2\)
35. According to Markownikoffs rule, negative part of reagent adds to that doubly bonded carbon atom of an unsymmetrical alkene which carries
a) less number of carbon atoms
b) more number of hydrogen atoms
c) less number of hydrogen atoms
d) no hydrogen atoms
36. Anti-Markownikoffs rule is followed in the presence of
a) hydrogen peroxide
b) benzoyl chloride
c) phosphorus pentoxide
d) anhydrous aluminium chloride
37. Which of the following is the best reagent to convert alcohol into alkyl chloride?
a) \(\mathrm{PCh}\)
b) cone. \(\mathrm{HCI}\)
c) PCIs
d) \(\mathrm{SOCh}\)
38. Which reaction is useful to convert lower alkanes into higher alkanes ?
a) Halogenation of alkene
b) Wurtz synthesis
c) Williamson's synthesis
d) Sandmeyer's reaction
39. When ethyl bromide" and methyl bromide separately undergo Wurtz reaction the products formed are respectively.
a) ethane and propane
b) ethane and methane
c) butane and ethane
d) propane and butane
40. Grignard reagent is useful in the preparation of
a) alkane
b) ethers
c) alcohols
d) all of these
41. Which of the following reagent does NOT represent the example of electrophilic substitution reaction?
a) \(\mathrm{Cl}_2\) and anhydrous \(\mathrm{FeCl}_3\)
b) Conc, \(\mathrm{H}_2 \mathrm{SO}_4\)
c) Nitrating mixture
d) Anhydrous \(\mathrm{CuCN}\) and \(\mathrm{NaCN}\)
42. Carbon-halogen bond is strongest in which among the following?
a) \(\mathrm{CH}_3 \mathrm{Cl}\)
b) \(\mathrm{CH}_3 \mathrm{Br}\)
c) \(\mathrm{CH}_3 \mathrm{~F}\)
d) \(\mathrm{CH}_3 \mathrm{I}\)
43. The atoms or groups which lose electron show effect and the atoms or groups which withdraw electrons show effect.
a) + I and \(-I\)
b) \(-\mathrm{I}\) and + I
44. Propene undergoes addition of \(\mathrm{HBr}\) in the presence of benzoyl peroxide to give __ as major product.
a) l-Bromobutane
b) 2-Bromobntane
c) 1-Bromopropane
d) 2-Bromopropane
45. Saytzeffs rule states that alkene is major product in dehydrogenation of an alkyl halide.
a) more substituted
b) less substituted
c) less branched
d) dehydrated
46. Which of the following is a primary alkyl halide?
a) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHCICH}_3\)
b) \(\mathrm{CH}_3 \mathrm{CHCICH}_2 \mathrm{CH}_3\)
c) \(\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2 \mathrm{CI}\)
d) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CCI}\)
47. The IUPAC name of tert-Butyl chloride is
a) 1-Chloro-2,2-dimethylethane
b) 1-Chloro-2-methylpropane
c) 2-Chlorobutane
d) 2-Chloro-2-methylpropane
48. Which of the following alkyl halides is hydrolysed by \(\mathrm{S}_{\mathrm{N}}{ }^1\) mechanism ?
a) \(\mathrm{CH}_3-\mathrm{Br}\)
b) \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{Br}\)
c) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CH}_2-\mathrm{Br}\)
d) \(\left(\mathrm{CH}_3\right)_3 \mathrm{C}-\mathrm{Br}\)
49. Which of the following alkyl halides is hydrolysed by \(\mathrm{S}_{\mathrm{N}}{ }^2\) mechanism ?
a) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{Br}\)
b) \(\mathrm{CH}_3 \mathrm{Br}\)
c) \(\mathrm{CH}_2=\mathrm{CHCH}_2 \mathrm{Br}\)
d) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CBr}\)
50. The product formed when ethyl bromide reacts with alcoholic silver cyanide is
a) ethyl isocyanide
b) propionitrile
c) methyl isocyanide d) acetonitrile
51. When 2-Chlorol:mtane is treated with alcoholic \(\mathrm{KOH}\), the major product formed is
a) butane
b) but-2-ene
c) but-l-ene
d) but-2-yne
52. Iodoform is used in medicine as an
a) antiseptic
b) anaesthetic agent
c) antipyretic
d) antibiotic
53. \(\mathrm{Mg}\) reacts with \(\mathrm{RBr}\) best in
a) dry ether
b) acetone
c) benzene
d) \(\mathrm{CCl}_4\)
54. The IUPAC name of the compound shown below is

a) 2-Bromo-6-chlorocyclohex-1-ene
b) 6-Bromo-2-chlorocyclohexene
c) 3-Bromo-l-chlorocyclohex-1-ene
d) 1-Bromo- 3-chlorocyclohex -2-ene
55. The number of possible optical isomers is given by the formula
a) \(4^n\)
b) \(3^a\)
c) \(1^{\mathrm{n}}\)
d) \(2^{\circ}\)
56. The racemic mixture of an optically active compound is
a) dextro rotatory
b) laevo rotatory
c) optically inactive
d) optically active
57. A molecule is said to be chiral if it
a) contains a plane of symmetry
b) contains a centre of symmetry
c) cannot be superimposed on its mirror
d) exists as cis-trans isomers
58. What is the sequence of priority for the following structure ?

a) \(\mathrm{NH}_2, \mathrm{ClCH}_2, \mathrm{CH}_3, \mathrm{H}\)
b) \(\mathrm{NH}_2, \mathrm{H}, \mathrm{ClCH}_2, \mathrm{CH}_3\),
c) \(\mathrm{CICH}_2, \mathrm{NH}_2, \mathrm{CH}_3, \mathrm{H}\)
d) \(\mathrm{CH}_3, \mathrm{NH}_2, \mathrm{CICH}_2, \mathrm{H}\)
60. Which of the following carbocations is least stable?

61. But-1-ene on reaction with \(\mathrm{HCl}\) in the presence of sodium peroxide yields
a) n-butyl chloride
b) isobutyl chloride
c) secondary butyl chloride
d) tertiary butyl chloride
62. Carbon atom in methyl carbocation contains how many pairs of electrons?
a) 8
b) 4
c) 3
d) 5

24. Alcohols, phenols and ethers
11.Alcohols, Phenols And Ethers
1. Which of the following alcohols is prepared by acid catalysed hydration of alkenes?
(a) Butan \(-1-o \ell\).
(c) ethanol
(d) methanol
2. Which of the following alcohols can be prepared by direct hydration of corresponding alkene in presence of \(50 \%\) sulphuric acid?
(a) Butan \(-1-o \ell\)
(b) Butan \(-2-o \ell\)
(c) 2 -Methylpropan \(-1-o \ell\)
(d) 2-Methylpropan \(-2-o \ell\)
3. Which of the following alcohols cannot be prepared by reduction of carbonyl compounds?
(a) Pentan \(-1-o \ell\)
(b) Pentan \(-2-o \ell\)
(c) 2 - Methylpentan \(-2-\mathrm{o} \ell\)
(d) 3 - Methylpentan - \(2-\mathrm{o}\) l
4. Which of the following conversions explains the acidic nature of alcohols?
(a) Ethanol \(\stackrel{\mathrm{H} \mathrm{Br}\}}{\longrightarrow}\) Bromoethane
(b) Ethanol \(\stackrel{\mathrm{N}_2}{\longrightarrow}\) Sodium ethoxide
(c) Ethanol \(\stackrel{\mathrm{Pa}_3}{\longrightarrow}\) Chloroethane
(d) Ethanol \(\stackrel{\mathrm{SOCl}_2}{\longrightarrow}\) Chloroethane
5. Which of the following compounds gives 3 -ethylpentan-3-0 \(\boldsymbol{\ell}\) by the action of ethyl magnesium iodide followed by acid hydrolysis?
(a) Propanone
(b) Butanone
(c) Pentan - 2 - one
(d) Pentan - 3 - one
6. Which of the following compound is obtained as major product on reaction of ethoxybenzene with nitrating mixture?
(a) 2 -Nitro ethoxybenzene
(b) 3 - Nitro ethoxybenzene
(c) 4 - Nitro ethoxybenzene
(d) Nitrobenzene
7. Benzyl phenyl ether reacts with hydrogen bromide to give
(a) benzyl bromide and phenol
(b) benzyl alcohol and bromobenzene
(c) benzyl bromide and bromobenzene
(d) benzyl alcohol and phenol
8. Ethers are considered as
(a) monoalkyl derivatives of water
(b) alkoxy derivatives of alkanes
(c) alkyl derivatives of fatty acids
(d) condensation products of acid and alcohol
9. Which of the following compounds is not isomeric with ethoxyethane?
(a) 1 - Methoxypropane
(b) 2 - Methoxypropane
(c) 2 - Methylpropan - 2 - ol
(d) 2 - Methylbutan - \(2-\mathrm{ol}\)
10. Which one of the following compounds dissolves in hot dilute sulphuric acid but does not reacts with sodium metal?
(a) ethyl bromide
(b) acetic acid
(c) ethyl alcohol
(d) diethyl ether
11. Which of the following alcohol will have the fastest rate of dehydration?

12. The phenol having lowest acidity is

13. Pyrogallol is
(a) 1,2-Dihydroxybenzene
(b) Methoxy benzene
(c) 2-Bromophen ol
(d) 1,2,3-Trihydroxybenzene
14. Which of the following is an excellent antiseptic?
(a) Benzaldehyde
(b) Benzyl alcohol
(c) Phenol
(d) Acetia acid
15. Carbolic acid is

16. Which of the following gives anisole?
(a) Phenyl and methyl chloride
(b) Benzyl alcohol and sodium hydroxide
(c) Aniline with nitrous acid
(d) Sodium phenoxide and methyl chloride
17. In the reaction

the compound \(\mathrm{X}\) is
(a) Ethyl alcohol
(b) Isopropyl alcohol
(c) Tert-butyl alcohol
(d) n-Propyl alcohol
18. Which of the following methods does not give phenol?

19. Which of the following groups increases the acidity of phenol?
(a) \(-\mathrm{CN}\)
(b) \(-X\) (halogen)
(c) \(-\mathrm{NO}_2\)
(d) all
20. The reaction
is an example of

(a) Schotten Baumann reaction
(b) Friedel Craft's reaction
(c) Etard reaction
(d) Perkin reaction
21. Vinyl carbinol is

22. Intramolecular hydrogen bonding is found in
(a) o-bromophenol
(b) o-nitrophenol
(c) m-nitrophenol
(d) p-nitrophenol
23. How many isomeric acyclic alcohols and ethers are possible for \(\mathrm{C}_4 \mathrm{H}_8 \mathrm{O}\) ?
(a) 3
(b) 4
(c) 5
(d) 7
24. Unknown compound \((X)\) on hydration by conc. \(\mathrm{H}_2 \mathrm{SO}_4\) gives \((\mathrm{Y})\). The compound \((\mathrm{Y}\) ) on oxidation gives acetone. The compound \((X)\) is
(a) \(\mathrm{CH}_3 \mathrm{CH}=\mathrm{CH}_2\)
(b) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\)
(c) \(\mathrm{CH}_3 \mathrm{CHOHCH}_3\)
(d) \(\mathrm{CH}_2=\mathrm{CH}_2\)
25. An example of benzylic alcohol is

26. Which of the following isomers of butanol has a chiral structure?
(a) \((\mathrm{CH} 3)_3 \mathrm{CH}_2 \mathrm{OH}\)
(b) \(\left(\mathrm{CH}_3\right)_2 \mathrm{CHCH}_2 \mathrm{OH}\)
(c) \(\mathrm{CH}_3 \mathrm{CH}(\mathrm{OH}) \mathrm{CH}_2 \mathrm{CH}_5\)
(d) \(\mathrm{CH}_3\left(\mathrm{CH}_2\right)_3 \mathrm{OH}\)
27. A compound \(\mathrm{X}\) with molecular formula \(\mathrm{C}_3 \mathrm{H}_8 \mathrm{O}\) can be oxidised to a compound \(Y\) with the molecular formula \(\mathrm{C}_3 \mathrm{H}_6 \mathrm{O}_2 \times \mathrm{X}\) is most likely to be
(a) Primary alcohol
(b) sec. alcohol
(c) aldehyde
(d) ketone
28. Raney nickel is a/an
(a) alloy of \(\mathrm{Al}\) and \(\mathrm{Ni}\) leached in sodium hydroxide solution
(b) alloy of \(\mathrm{Al}\) and Fe leached in caustic soda solution
(c) alloy of \(\mathrm{Fe}\) and \(\mathrm{Co}\) leached in soda bicarbonate
(d) all of these
29. Phenol is less acidic than
(a) Ethanol
(b) Methanol
(c) o-Nitrophenol
(d) p-Methyl phenol
30. The increasing orded of acidity among phenol: p-methyl, m-nitrophenol and p-nitrophenol is
(a) phenol, p-methyl phenol, p-nitriphenol, m-nitrophenol
(b) p-methyl phenol, phenol, m-nitrophenol, p-nitriphenol
(c) p-nitrophenol, m-nitriphenol, phenol, p-methyl phenol
(d) m-nitrophenol, p-nitriphenol, phenol, p-methyl phenol
31. An alcohol having molecular formula \(\mathrm{C}_6 \mathrm{H}_{11} \mathrm{OH}\) on dehydration gives an alkene, which on oxidation yield a mixture of ketone and acid. The alcohol is

32. The correct IUPAC name of the compound \(\mathrm{CH}_3 \mathrm{CH}\left(\mathrm{C}_2 \mathrm{H}_5\right) \mathrm{CH}_2 \mathrm{CH}_2(\mathrm{OH}) \mathrm{CH}_3\) is
(a) 2-Ethylpentan-4-ol
(b) 2-Hyxroxy-4-methyl pentane
(c) 4-Ethylpentan-2-ol
(d) 4-Methylhexan-2-ol
33. Phenol can be industrially prepared from cumene. It is
(a) Isopropyl benzene
(b) o-Dimethylbenzene
(c) Phenyl acetate
(d) 2-Acetoxylbenzoic acdi
34. Consider the following alkyl halides
1) \(\left(\mathrm{CH}_3\right)_3 \mathrm{CBr}\)
2) \(\mathrm{CH}_3 \mathrm{Br}\)
3) \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{Br}\)
4) \(\mathrm{CH}_3 \mathrm{CHBrCH}_3\)
Arrange these alkyl halides in decreasing order of reactivity in Williamson's reaction.
(a) \(2>3>4>1\)
(b) \(4>3>2>1\)
(c) \(1>4>3>2\)
(d) \(1>2>3>4\)
35. Which one of the following compound is most acidic

36. In the reaction

Which of the following compounds will be formed

37. During dehydration of alcohols to alkenes by heating with conc. \(\mathrm{H}_2 \mathrm{SO}_4\), the initial step is
(a) formation of an ester
(b) protonation of alcohol molecule
(c) formation of carbocation
(d) elimination of water
38. Which of the following alcohols cannot be prepared by the action of a suitable Grignard reagent as an aldehyde or a ketone followed by hydrolysis?
(a) Ethyl alcohol
(b) Isopropyl alcohol
(c) n-Propyl alcohol
(d) Methanol
39. The best reagent to convert pent-2-en-2-ol into pent-3-en-2-one is
(a) acidic permagnate
(b) acidc dichromate
(c) chromic anhydride in glacial acetic acid
(d) pyridinium chloro chromate
40. Arrange the following compounds according to decreasing boiling points

(a) (IV) \(>\) (III) \(>\) (II) \(>\) (I)
(b) (III) \(>\) (IV) \(>\) (II) \(>\) (I)
(c) (I) \(>\) (II) \(>\) (III) \(>\) (IV)
(d) (III) \(>\) (II) \(>\) (I) \(>\) (IV)
41. In the following sequence of reactions

The compound ' \(\mathrm{D}\) ' is
(a) n-butyl alcohol
(b) n-propyl alcohol
(c) propanal
(d) butanal
42. Hydrolysis of 2-bromo-2-methylbutane by \(\mathrm{SN}^1\) mechanism gives mainly
(a) 3-methylbutan-2-ol
(b) 2-methylbutan-2-ol
(c) 2,2-dimethylpropan-2-ol
(d) 2-methylbutan-1-ol
43. In the following reaction, \(C\) is

(a) \(\mathrm{H}_2 \mathrm{C}=\mathrm{CH}\)
(b) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\)
(c) \(\mathrm{C}_2 \mathrm{H}_3-\mathrm{OC}_2 \mathrm{H}_5\)
(d) \(\mathrm{C}_2 \mathrm{H}_5-\mathrm{OS} \mathrm{O}_3 \mathrm{H}\)
44. The compound which is not isomeric with diethyl ether is
(a) n-Propyl methyl ether
(b) butan-1-ol
(c) 2-Methylpropan-2-ol
(d) Butanone
45. The most suitable reagent for the conversion of primary alcohol into aldehyde with the same number of carbon is
(a) acidic \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\)
(b) acidified \(\mathrm{KMnO}_4\)
(c) Pyridinium chlorochromate
(d) \(\mathrm{CrO}_3\)
46. A compound ' \(X\) ' undergoes reduction with \(\mathrm{LiAlH}_4\) to yield ' \(Y\) '. when vapours of ' \(Y\) ' are passed over freshly reduced copper at \(300^{\circ} \mathrm{C}\), ' \(X\) ' is formed. What is ' \(Y\) '?
(a) \(\mathrm{CH}_3 \mathrm{COCH}_3\)
(b) \(\mathrm{CH}_3 \mathrm{CHO}\)
(c) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\)
(d) \(\mathrm{CH}_3 \mathrm{OCH}_3\)
47. n-Propyl alcohol and isopropyl alcohol can be chemically distinguished by which reagent?
(a) \(\mathrm{PCl}_5\)
(b) reduction
(c) oxidation with potassium dichromate
(d) ozonolysis
48. When \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{COOH}\) is reduced with \(\mathrm{LiAlH}_4\), the compound obtained will be
(a) \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{COOH}\) (b) \(\mathrm{CH}_2=\mathrm{CH}-\mathrm{CH}_2 \mathrm{OH}\)
(c) \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CH}_2 \mathrm{OH}\) (d) \(\mathrm{CH}_3-\mathrm{CH}_2-\mathrm{CHO}\)
49. Which type of carbocation is formed as an intermediate during the dehydration 4,4-dimethylpentanol?
(a) \(1^0\)
(b) \(2^0\)
(c) \(3^0\)
(d) All the three
50. The relative acidic character of \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}\), \(\left(\mathrm{CH}_3\right)_2 \mathrm{CHOH}\) and \(\left(\mathrm{CH}_3\right)_3 \mathrm{COH}\) can be explained on the basis of
(a) resonance
(b) inductive effect
(c) hyperconjugation
(d) hybridisation

25. Aldehydes ketones and carboxylic acids
1. Which of the following carbonyl compounds undergoes aldol condensation?
(a) Benzaldehyde
(b) Benzophenone
(c) Acetophenone
(d) tert-Butyl phenyl ketone
2. Which of the following carbonyl compounds undergoes self redox reaction in presence of concentrated base?
(a) 3-Methylpentanal
(b) 2-Chlorobutanal.
(c) 2, 2-Dimethylpropanal
(d) tert-butyl methyl ketone.
3. The smell of bitter almond is given by the compound.
(a) Benzoic acid
(b) Benzaldehyde
(c) vanillin
(d) cinnamaldehyde
4. Which of the following will not give yellow precipitate when treated with \(\mathrm{NaOH}\) and \(\mathrm{I}_2\) ?
(a) 3-Methylbutan-2-one
(b) 2-methylpentan-3-one
(c) Propanone
(d) Hexan-2-one
5. A \(\beta\)-hydroxyl carbonyl compound is obtained by the action of \(\mathrm{NaOH}\) on
(a) \(\mathrm{HCHO}\)
(b) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CHO}\)
(c) \(\mathrm{CR}_3 \mathrm{CHO}\)
(d) \(\mathrm{CH}_3 \mathrm{CHO}\)
6. Para aldehyde is obtained by polymerization of
(a) \(\mathrm{HCHO}\)
(b) \(\mathrm{CH}_3 \mathrm{CHO}\)
(c) \(\mathrm{CH}_3 \mathrm{OH}\)
(d) \(\mathrm{CH}_3 \mathrm{CH}_2-\mathrm{CHO}\)
7. Schiff's reagent gives pink color with
(a) acetone
(b) acetic acid
(c) acetaldehyde
(d) methyl acetate.
8. Dry distillation of a mixture of calcium formate and calcium acetate gives.
(a) formaldehyde
(b) acetaldehyde
(c) acetone
(d) acetophenone.
9. Metaldehyde is a
(a) tetramer of acetaldehyde
(b) dimer of acetone
(c) trimer of acetaldehyde
(d) trimer of formaldehyde
10. The reaction of \(\mathrm{C}_6 \mathrm{H}_8 \mathrm{CH}=\mathrm{CHCHO}_{\text {with }} \mathrm{LiAlH}_4\) gives
(a) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{OH}\)
(b) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CH}_2 \mathrm{CHO}\)
(c) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}=\mathrm{CHCH}_2 \mathrm{OH}\)
(d) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{CH}_2 \mathrm{CHOHCH}_3\)
11. A mixture of sodium benzoate and sodalime on heating yields
(a) methane
(b) benzene
(c) sodium benzoate
(d) calcium benzoate.
12. Which -is the strongest acid?
(a) \(\mathrm{CH}_3 \mathrm{COOH}\)
(b) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{COOH}\)
(c) \(\mathrm{CH}_3 \mathrm{CCOOH}\)
(d) \(\mathrm{ClCH}_2 \mathrm{COOH}\)
13. Benzaldehyde when treated with alkaline \(\mathrm{KMnO}_4\) yields
(a) Benzyl alcohol
(b) Benzoic acid
(c) \(\mathrm{CO}_2\) and \(\mathrm{H}_2 \mathrm{O}\)
(d) Salicylic acid
14. Acetonitrile on acidic hydrolysis gives.
(a) \(\mathrm{HCOOH}\)
(b) \(\mathrm{CH}_3 \mathrm{NC}\)
(c) \(\mathrm{CH}_3 \mathrm{COONa}\)
(d) \(\mathrm{CH}_3 \mathrm{COOH}\)
15. The organic compounds \(A\) and \(B\) reacts with sodium metal and liberates hydrogen gas. A and \(B\) reacts together to give ethyl acetate. The \(A\) and \(B\) are.
(a) \(\mathrm{CH}_3 \mathrm{COOH}\) and \(\mathrm{C}_2 \mathrm{H}_3 \mathrm{OH}\)
(b) \(\mathrm{HCOOH}\) and \(\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}\)
(c) \(\mathrm{CH}_3 \mathrm{COOH}\) and \(\mathrm{HCOOH}\)
(d) \(\mathrm{CH}_3 \mathrm{COOH}\) and \(\mathrm{CH}_3 \mathrm{OH}\)
16. The compound used as synthetic lemonade is
(a) tartaric acid
(b) benzoic acid
(c) acetic acid
(d) citric acid
17. o-halogentaion of carboxylic acid is called.
(a) Gattermann reaction
(b) Riemer-Tiemer reaction
(c) Sandmeyer's reaction
(d) HVZ reaction
18. The strongest acid is

19. The acid which does not undergo \(\mathrm{HVZ}\) reaction is
(a) acetic acid
(b) formic acid
(c) propanoic acid
(d) 2-methyl propanoic acid
20. An organic ester is
(a) Kerosene oil
(b) coconut oil
(c) soap
(d) glycerine
21. Propionic acid on bromination gives two isomeric 2-bromo propionic acids, they are
(a) optical isomers
(b) cis-trans isomers
(c) Position isomers
(d) chain isomers
22. Carbonyl compound in which one valency of carbonyl carbon is statisfied by \(\mathrm{H}\) atom is
(a) Aldehyde
(b) Ketone
(c) Carboxylic acid
(d) Acid amide
23. First oxidation product of secondary alcohol is
(a) Aldehydes
(b) Alkanone
(c) Acetone
(d) Alkanes
24. Common name of \(\mathrm{CH}_3-\mathbf{C H}=\mathbf{C H}-\mathbf{C H O}\) is
(a) Butenal
(b) Crotanaldehyde
(c) Acrolin
(d) Mesityl oxide
25. IUPAC name of

(a) 3-cyclobutyneyl propanal
(b) 3-cyclobut-1-enyl propanal
(c) 3-cyclobutylpropanal
(d) 3-cyclobut-2-enylpropanal
26.

(a) salicyaldehyde
(b) vanillin
(c) o-Tolualdehyde
(d) phthaldehyde
27. Most effectively aldehydes are prepared from primary alcohols by using
(a) Acidic \(\mathrm{KMnO}_4\)
(b) \(\mathrm{K}_2 \mathrm{Cr}_2 \mathrm{O}_7\) and dil \(\mathrm{H}_2 \mathrm{SO}_4\)
(c) dil \(\mathrm{HNO}_3\)
(d) \(\mathrm{PCC}\)
28. Ozonoide of \(\alpha\)-butylene on heating with \(\mathrm{Zn}\) and water produce
(a) only acetalhyde
(b) only formaldehyde
(c) mixture of acetaldehyde and methanal
(d) mixture of methanal and propanal
29. Ozonolysis product of

heating with Zn dust and water produes
(a) Hexanone and formaldehyde
(b) cyclohexanone only
(c) Cyclohexanone and methanal
(d) Capraldehyde and methanal
30. Dry distillation product of calcium propionate is
(a) Acetone
(b) propionaldehyde
(c) propanone
(d) diethylketone
31. Reducing agent used in Stephens reaction is
(a) \(\mathrm{Sn}\) and conc. \(\mathrm{HCl}\)
(b) \(\mathrm{SnCl}_2\) and dil. \(\mathrm{HCl}\)
(c) \(\mathrm{CrO}_2 \mathrm{Cl}_2\)
(d) DIBAl - H
32. By Etard reaction aldehydes are preapred from
(a) Aromatic hydrocarbons by halogenation and hydrolysis
(b) Aromatic hydrocarbons, \(\mathrm{CO}_2\) and \(\mathrm{HCl}\) in \(\mathrm{CrO}_3\)
(c) Aromatic hydrocarbons, acetic anhydride in \(\mathrm{CrO}_3\)
(d) Aromatic hydrocarbons, chromyl chloride in \(\mathrm{CS}_2\)
33. Formylation of benzene is performed by using
(a) \(\mathrm{CO}\) and \(\mathrm{HCl}\)
(b) Formic acid
(c) Formic amide
(d) Formonitrile
34. Benzophenone is obtained by Friedel Crafts reaction from
(a) Benzene and Acetyl chloride
(b) Benzene and Benzoyl chloride
(c) Benzene and chlorobenzene
(d) Benzene and acetophenone
35. Correct statement about the aldehydes and ketones is
(a) + I effect of alkyl group linked to carbonyl carbons increase their reactivity
(b) As the steric effect of alkyl groups increases reactivity of aldehydes and ketones increases
(c) Carbonyl carbon in aromatic aldehydes and ketones is more electron deficient due to electron attracting resonance effect
(d) aromatic aldehydes are less reactive than aliphatic aldehydes
36. Mandelonitrile is obtained from
(a) Benzaldehyde and \(\mathrm{HCN}\)
(b) Acetone and ammonia
(c) Acetaldehyde and \(\mathrm{HCN}\)
(d) Acetaldehyde and hydrazine
37. Tertiary alcohols are obtained from
(a) formaldehyde and R-Mg-X
(b) propanal and \(\mathrm{R-Mg}-\mathrm{X}\)
(c) alkanone and R-Mg-X
(d) Alkanal and \(\mathrm{NH}_3\)
38. Number of methylene groups in urotropin is
(a) 2
(b) 4
(c) 5
(d) 6
39. Aldehydes are converted into oximes by
(a) \(\mathrm{NH}(\mathrm{OH})_2\)
(b) \(\mathrm{HO}-\mathrm{NH}_2\)
(c) \(\mathrm{NH}_3\)
(d) \(\mathrm{NH}_2-\mathrm{NH}_2-\mathrm{OH}\)
40. One molecule of \(\mathrm{LiAlH}_4\) can reduce molecules of aldehydes or ketones.
(a) 1
(b) 2
(c) 3
(d) 4
41. Pinacol is
(a) Primary diol
(b) Secondary diol
(c) Tertiary diol
(d) Geminal tertiary diol
42. Aldehydes show reducing properties due to presence of
(a) \(\alpha\)-Hydrogen
(b) Carbonyl group
(c) \(\mathrm{H}\)-atom on carbonyl carbon
(d) Absence of \(-\mathrm{OH}\) group on \(-\stackrel{\mathrm{|}}{\mathrm{C}}=\mathrm{O}\)
43. Cannizzaro's reaction is not shown by
(a) Methanal
(b) Benzaldehyde
(c) Ethanal
(d) Isobutyraldehyde
44. Aromatic aldehydes undergo types of reactions of aromatic ring.
(a) Electrophilic substitution
(b) Nucleophilic substitution
(c) Electrophilic addition
(d) Nucleophilic addition
45. Tricarboxylic acid is
(a) Glutaric acid
(b) Citric acid
(c) Tartaric acid
(d) Malonic acid
46. Correct IUPAC name of

(a) 3-carboxyl ethyl pentane-1,5-dioic acid
(b) 3-carboxylmethyl pentane-1,5-dioic acid
(c) 3-carboxyl pentane-1,5-dioic acid
(d) propane-1,2,3-tricarboxylic acid
47. Boiling points of carboxylic acids are higher than alcohols due to
(a) formation of dimer through strong intermolecular hydrogen bonding
(b) strong dipole-dipole interaction
(c) their large size
(d) strong bond between carbon and oxygen
48. Carboxylic acids on heating with thionyl chloride produce
(a) Acyl chlorides
(b) Acetyl chloride
(c) Alkyl chloride
(d) Hydrogen chloride
49. Fisher esterification is
(a) Electrophilic addition
(b) Nucleophilic addition
(c) Electrophilic substitution
(d) Nucleophilic substitution
50. Phthalimide is obtained by strong heating ammonia with
(a) phthalic anhydride (b) phthalic acid
(c) phthaladehyde
(d) o-toluic acid

26. compounds containing nitrogen
1. Which of the following amines cannot be prepared by Gabriel phthalimide synthesis?
(a) sec - propylamine
(b) tert - butylamine
(c) 2 - Phenylethylamine
(d) N - Methylbenzylamine
2. Which of the following compounds has highest boiling point?
(a) Ethane
(b) Ethanoic acid
(c) Ethanol
(d) Ethanamine
3. Identify the statement about the basic nature of amines.
(a) Alkylamines are weaker bases than ammonia.
(b) Arylamines are stronger bases than alkylamines.
(c) Secondary aliphatic amines are stronger bases than primary aliphatic amines
(d) Tertiary aliphatic amines are weaker bases than arylamines.
4. The compounds \(\mathrm{A}, \mathrm{B}\) and \(\mathrm{C}\) react with methyl iodide to give finally quaternary ammonium iodides. Only C gives carbylamines test while only \(A\) form yellow oily compound on reaction with nitrous acid. The compounds \(A, B\) and \(C\) are respectively.
(a) Butan - 1 - amine, \(\mathrm{N}\) - Ethylethanarnine and \(\mathrm{N}, \mathrm{N}\) - Dimethylethanamine.
(b) N-Ethylethanamine, N, N - Dimethylethanamine and Butan - 1 - amine.
(c) N, N-Dimethylethanamine, \(\mathrm{N}\) - Ethylethanamine and Butan - 1 - amine.
(d) N- Ethylethanamine, Butan - 1 - amine and N- Ethylethanamine.
5. Which of the following amines is most basic in nature?
(a) 2,4-Dichloroaniline
(b) 2, 4 - Dimethylaniline
(c) 2,4-Dinitroaniline
(d) 2, 4-Dibromoaniline
6. How many moles of methyl iodide are required to convert ethylamine, diethylamine and triethylamine into quaternary ammonium salt, respectively?
(a) 1,2 and 3
(b) 2,3 and 1
(c) 3,2 and 1
(d) 3,1 and 2
7. Which of the following amines does not undergoes acetylation?
(a) \(\mathrm{t}\) - butylamine
(b) ethylamine
(c) diethylamine
(d) triethylamine
8. Acetoxime on catalytic reduction gives
(a) Acetic acid
(b) acetic anhydride
(c) ethyl amine
(d) isopropylamine
9. n-Propylamine can be prepared by catalytic reduction of
(a) n-propyl cyanide
(b) propionaldoxime
(c) acetoxime
(d) nitroethane
10. Identify the compound ' \(B\) ' in the following series of reactions.

11. Secondary nitro alkanes react with nitrous acid to form
(a) red solution
(b) blue solution
(c) green solution
(d) yellow solution
12. Chloropicrin is used as
(a) antiseptic
(b) antibiotic
(c) insecticide
(d) anaesthetic.
13. Which one of the following compounds will show nitro-acinitro tautomerism?

14. Nef carbonyl synthesis is given by

15. Which one of the following nitroalkanes will give nitrolic acid with \(\mathrm{NaNO}_2 /\) conc. \(\mathrm{H}_2 \mathrm{SO}_4^{\text {? }}\)

16. Arrange given amines in decreasing order of their basicity

(a) I \(>\) II \(>\) III
(b) I \(>\) III \(>\) II
(c) III \(>\) II \(>\) I
(d) II \(>\) I \(>\) III
17. In the given reaction

[X] will be
(a) \(\mathrm{H}_2 / \mathrm{Ni}\)
(b) \(\mathrm{Sn} /\) conc. \(\mathrm{HCl}\)
(c) \(\mathrm{LiAlH}_4\)
(d) All of these
18. In the given reaction

[X] will be
(a) \(\mathrm{Sn} /\) conc. \(\mathrm{HCl}\)
(b) \(\mathrm{Sn} /\) conc. \(\mathrm{HNO}_3\)
(c) \(\mathrm{Sn} /\) conc. \(\mathrm{H}_2 \mathrm{SO}_4\)
(d) \(\mathrm{Sn} / \mathrm{CH}_3 \mathrm{COOH}\)
19. Which one of the following is aliphatic primary amine?
(a) \(\mathrm{CH}_3-\mathrm{NH}-\mathrm{C}_6 \mathrm{H}_5\)
(b) \(\mathrm{C}_6 \mathrm{H}_5-\mathrm{NH}_2\)
(c) \(\mathrm{C}_6 \mathrm{H}_5-\mathrm{CH}_2-\mathrm{CH}_2-\mathrm{NH}_2\)
(d) \(\mathrm{C}_6 \mathrm{H}_5-\mathrm{NH}-\mathrm{C}_6 \mathrm{H}_5\)
20. Ammonolysis of alcohol will NOT give
(a) Primary amine
(b) Secondary amine
(c) quaternary ammonium hydroxide
(d) Tertiary amine
21. In the Hoffmann-Bromamide rearrangement, intermediate compounds are

22. In the given reaction sequence

23. In Gabriel synthesis; amine is always
(a) Aliphatic primary amine
(b) Aliphatic secondary amine
(c) Aromatic primary amine
(d) Aromatic secondary amine
24. \(\mathrm{Br}_2 / \mathrm{NaOH}\) gives bromination reaction with amines at
(a) Nitrogen having hydrogen
(b) \(\alpha\)-carbon having hydrogen
(c) Nitrogen having no \(\alpha\)-hydrogen
(d) \(\alpha\)-carbon having no \(\alpha\)-hydrogen
25. Nitrosoamine test is given by
(a) Primary amines
(b) Primary amines
(c) Aliphatic as well as aromatic secondary amines
(d) Quaternary ammonium halides
26. Which one of the following will NOT give carbylamine reaction?

27. Which of the following orders amongst amines in the gaseous state is true regarding the basic nature of \(\mathrm{NH}_2\) group?
(a) \(\mathrm{CH}_3 \mathrm{NH}_2>\left(\mathrm{CH}_3\right)_2 \mathrm{NH}>\left(\mathrm{CH}_3\right)_3 \mathrm{~N}\)
(b) \(\mathrm{CH}_3 \mathrm{NH}_2>\left(\mathrm{CH}_3\right)_2 \mathrm{NH}<\left(\mathrm{CH}_3\right)_3 \mathrm{~N}\)
(c) \(\mathrm{CH}_3 \mathrm{NH}_2<\left(\mathrm{CH}_3\right)_2 \mathrm{NH}>\left(\mathrm{CH}_3\right)_3 \mathrm{~N}\)
(d) \(\mathrm{CH}_3 \mathrm{NH}_2<\left(\mathrm{CH}_3\right)_2 \mathrm{NH}<\left(\mathrm{CH}_3\right)_3 \mathrm{~N}\)
28. Amongst the following, the most basic compund is
(a) benzylamine
(b) aniline
(c) acetanilide
(d) p-nitroaniline
29. Which of the following orders is correct regarding the basic strength of substituted aniline?
(a) p-nitroaniline \(>\) p-aminobenzaldehyde \(>\) p-bromoaniline
(b) p-nitroaniline \(<\) p-bromoaniline \(<\) p-aminobenzaldehyde
(c) p-nitroaniline \(<\) p-aminobenzaldehyde \(<\) p-bromoaniline
(d) p-nitroaniline \(>\) p-aminobenzaldehyde \(<\) p-bromoaniline
30. The number of resonating structures of aniline is
(a) 2
(b) 3
(c) 4
(d) 5
31. Tertiary amine contains

32. Which of the following is Sandmeyer reaction?
(a) \(2 \mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl} \stackrel{\mathrm{Cl}_2 \mathrm{Cl}_2 / \mathrm{HCl}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5+\mathrm{Cl}_2\)
(b) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{~N}_2 \mathrm{Cl} \stackrel{\mathrm{Cu}_2 \mathrm{O}_2 / \mathrm{HCl}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5 \mathrm{Cl}_2+\mathrm{N}_2\)
(c) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{OH} \stackrel{\text { Zn dust }}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5+\mathrm{ZnO}\)
(d) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NO}_2+6[\mathrm{H}] \stackrel{\mathrm{Sa} / \mathrm{HCl}}{\longrightarrow} \mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2+2 \mathrm{H}_2 \mathrm{O}\)
33. The compound which on reaction with aqueous nitrous acid at low temperature produces an oily nitrosoamine is
(a) methylamine
(b) ethylamine
(c) diethylamine
(d) triethylamine
34. On adding KI to benzenediazonium chloride, the product obtained is
(a) benzene
(b) 1-4-diiodobenzene
(c) iodobenzene
(d) 1,3,5-triiodobenzene
35. Which of the following reagents can convert benzenediazonium chloride into benzene?
(a) Water
(b) Acid
(c) Hypophosphorous acid
(d) \(\mathrm{HCl}\)
36. Which of the following undergoes diazotization?
(a) \(\mathrm{CH}_3 \mathrm{NH}_2\)
(b) \(\mathrm{C}_6 \mathrm{H}_5 \mathrm{NH}_2\)
(c) \(\mathrm{CH}_3 \mathrm{CONH}_2\)
(d) \(\mathrm{CH}_3 \mathrm{~N}\left(\mathrm{CH}_3\right)_2\)
37. Hinsbert's reagent is
(a) phenylisocyanide
(b) benzenesulphonyl chloride
(c) p-toluenesulphonic acid
(d) o-dichlorobenzene
38. Which of the following can distinguish the three amines, viz., primary, secondary and tertiary?
(a) Azo-dye test
(b) Hinsberg reagent
(c) Carbylamine test
(d) Acetyl chloride
39. Hofmann's method to separate amines in a mixture uses the reagent
(a) benzenesulphonyl chloride
(b) diethyl oxalate
(c) benzeneisocyanide
(d) p-toluenesulphonic acid
40. IUPAC name of the compound

(a) Methylpropamine
(b) 2-Methylbutan-1-amine
(c) 2-Methylpropan-1-amine
(d) 2-Methylethan-1-amine
41. Isomerism shown by amines is
(a) chain
(b) position
(c) functional
(d) all of these
42. The reduction of which of the following compounds would yield secondary amine?
(a) alkyl nitrile
(b) carbylamine
(c) primary amine
(d) sec nitro compound
43. The conversion of alky cyanides into primary amines by sodium and alcohol is called
(a) Ammonolysis
(b) Sabatier-Sanderson's reduction
(c) Mendius reduction
(d) Clemmensen's reduction
44. Acetylation in amines is replacement of ' \(H\) ' of \(\mathrm{NH}_2\) or \(-\mathrm{NH}\) by
(a) \(-\mathrm{CH}_2 \mathrm{COCl}\) group
(b) \(-\mathrm{CH}_2 \mathrm{CO}\) group
(c) \(-\mathrm{COCH}_3\) group
(d) \(-\mathrm{COOCH}_3\) group
45. Carbylamine test is performed in alcoholic \(\mathrm{KOH}\) by heating a mixture of
(a) chloforom and silver powder
(b) trihalogenated methane and primary amine
(c) an alkyl halide and primary amine
(d) an alkyl cyanide and primary amine
46. Valency of \(\mathrm{N}\)-atom in amines is
(a) 5
(b) 2
(c) 3
(d) 4
47. Optically active amines among the following is
(a) methyl amine
(b) dimethyl amine
(c) trimethyl amine
(d) sec-butyl amine
48. Quaternary ammonium salt contains how many co-ordinate bonds?
(a) One
(b) Two
(c) Three
(d) Zero
49. Amines when react with water from
(a) acids
(b) bases
(c) salts
(d) ethers
50. Hybridisation of nitrogen atom in amines is
(a) \(\mathrm{NaOH}\)
(b) \(\mathrm{sp}^3\)
(c) \(\mathrm{H}_2 \mathrm{SO}_4\)
(d) unhybridised

27. Biomolecules
1. Milk sugar is
(a) sucrose
(b) lactose
(c) maltose
(d) glucose
2. The carbohydrate used for silvering of mirror is
(a) fructose
(b) starch
(c) glucose
(d) cellulose
3. Raffinose is a
(a) monosaccharide
(b) disaccharide
(c) trisaccharide
(d) polysaccharide
4. Which one of the following is NOT produced by human body.
(a) DNA
(b) hormones
(c) enzymes
(d) vitamins
5. A biological catalyst is essentially
(a) an amino acid
(b) an enzyme
(c) a nitrogen molecule
(d) a carbohydrate
6. Night blindness is due to the deficiency of
(a) \(\operatorname{vitamin} A\)
(b) vitamin \(B\)
(c) vitamin \(\mathrm{C}\)
(d) vitamin D
7. Which of the following contains the element, cobalt?
(a) vitamin c
(b) vitamin \(B_{12}\)
(c) chlorophyll
(d) haemoglobin
8. Which of the following vitamins is water soluble?
(a) \(\mathrm{A}\)
(b) \(B\)
(c) \(\mathrm{E}\)
(d) D
9. Which one of the following is not a constituent of RNA?
(a) Ribose
(b) Uracil
(c) Thymine
(d) Phosphate
10. DNA is a polymer of units of
(a) sugars
(b) ribose
(c) amino acids
(d) nucleotides
11. Which one of the following molecules will form zwitter ion?
(a) \(\mathrm{CH}_3 \mathrm{COOH}\)
(b) \(\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{NH}_2\)
(c) \(\mathrm{CCl}_3 \mathrm{NO}_2\)
(d) \(\mathrm{NH}_2 \mathrm{CH}_2 \mathrm{COOH}\)
12. In metabolic process the maximum energy is given by
(a) carbohydrates
(d) proteins
(c) vitamins
(d) fats
13. Insulin is
(a) harmone
(b) antibiotic
(c) antiseptic
(d) vitamin
14. The secondary structure of a protein is determined by
(a) co-ordinate bond
(b) covalent bond
(c) ionic bond
(d) hydrogen bond
15. In maltose, glycosidic linkage is present between the two glucose units at positions
(a) 1,2
(b) 1,1
(c) 1,3
(d) 1,4
16. Vitamin \(B_2\) is also known as
(a) riboflavin
(b) thiamine
(c) nicotinamide
(d) pyridoxine
17. The sugar present in DNA is
(a) deoxyribose
(b) ribulose
(c) glucose
(d) ribose
18. Sucrose molecule consists of
(a) a glucofuranose and a fructopyranose
(b) a glucofuranose and a fructofuranose
(c) a glucopyranose and a fructopyranose
(d) a glucopyranose and a fructofuranose
19. Which of the following statement is not correct about DNA molecule?
(a) It has double helix structure
(b) It serves as hereditary material
(c) The two DNA strands are exactly similar
(d) Its replication is called semi-conservative mode of replication.
20. The number of \(\mathrm{sp}^2\) and \(\mathrm{sp}^3\) hybridized carbon atoms in fructose are respectively.
(a) 4 and 2
(b) 2 and 4
(c) 1 and 5
(d) 5 and 1
21. A sesquiterpene, abscisic acid contains how many isoprene units?
(a) Two
(b) Three
(c) Four
(d) Five
22. Cholesterol is an example of
(a) zoosterols
(b) phytosterol
(c) mycosterols
(d) glycerols
23. A terpene, caryophyllene is found in
(a) oil of turpentine
(b) oil of roses
(c) oil of ginger
(d) oil of cloves
24. Which of the following substances does not fall in the category of carbohydrates?
(a) Sugars
(b) Starch
(c) Glycol
(d) Cellulose
25. Which of the following does not belong to the category of hexoses?
(a) Glucose
(b) Fructose
(c) Galactose
(d) Ribose
26. Which of the following statements is not correct?
(a) Symbols D- and L-before the name of monosacharides refer to the direction of rotation of the light
(b) Pyranose has a six-membered ring structure
(c) Furanose has a five-membered ring structure
(d) Pyranose and furanose are the examploes of hemiketal
27. Maltose is formed by the union of
(a) one molecule of glucose and one molecule of fructose
(b) one molecule of glucose and one molecule of fructose
(c) two molecule of glucose
(d) two molecule of galactose
28. Which of the following sugars has the largest relative sweetness with respect to sucrose?
(a) Glucose
(b) Fructose
(c) Galactose
(d) Maltose
29. Invert sugar is a mixture of
(a) glucose and sucrose
(b) glucose and fructose
(c) glucose and maltose
(d) lactose and maltose
30. Monomer of cellulose is
(a) lactose
(b) maltose
(c) fructose
(d) glucose
31. Which of the following statements is not correct?
(a) Polysaccharides are also known as glycans
(b) Glycogen, starch and cellulose are the examples of homopolysaccharids
(c) Amylose is a branched polymer of \(\alpha\)-glucose
(d) Starch exists in two polymorphic forms \(\alpha\) amylose and amylopectin
32. Which of the following statements is not correct?
(a) In nature, about 20 amino acids occur in proteins
(b) The human body can synthesize all the 20 amino acids occuring in proteins
(c) The simplest amino acid is glycine
(d) There are ten essential amino acids
33. The number of amino acids in insulin is
(a) 31
(b) 41
(c) 51
(d) 61
34. The number of amino acid units in haemoglobin is
(a) 554
(b) 564
(c) 574
(d) 584
35. Which of the following statements is not correct?
(a) The tertiary structure of proteins is three dimensional
(b) In globular proteins, nearly allthe hydrophobic groups are hidden inside and the polar groups are present on the surface resulting into a spheroidal shape
(c) Only hydrogen bonds are involved in the tertiary structure of proteins
(d) Globular proteins are insoluble in water
36. Which of the following statements is not correct?
(a) A peptide bond is \(-\mathrm{CO}-\mathrm{NH}-\)
(b) Each polypeptide has one C-terminal and the other \(\mathrm{N}\)-terminal
(c) The aminoa cid sequence of a protein determines the function of the protein
(d) The union of two amino acids produces two peptide linkages
37. Nucleic acids are
(a) small molecules
(b) polymeric in nature
(c) the compounds of \(\mathrm{C}, \mathrm{H}\) and \(\mathrm{O}\)
(d) essentially proteins
38. Which of the following bases is not common to DNA and RNA?
(a) Adenine
(b) Guanine
(c) Thymine
(d) Cytosine
39. Which of the following bases is present in RNA only?
(a) Adenine
(b) Guanine
(c) Uracil
(d) Thymine
40. Thymine is held by two hydrogen bonds with the base
(a) guanine
(b) cytosine
(c) thymine
(d) adenine
41. A cell membrane is made up of
(a) lipid
(b) phospholipid
(c) bilayer of phospholipid molecule
(d) only protein
42. Which of the following is not a lipid?
(a) Oils
(b) Waxes
(c) Cholesterol
(d) Glycerol
43. The constitutents of phospholipids are
(a) glycerol and fatty acids
(b) glycerol and phosphate
(c) glycerol, phosphate, two fatty acids and one alcoholic compound
(d) glycerol phosphate, two fatty acids and one alcoholic compound
44. Biochemical reactions which can take place in the absence of oxygen are called
(a) glycolysis
(b) aerobic
(c) metlabolic
(d) anaerobic
45. Molasses contain about 50 per cent of
(a) starch
(b) sucrose
(c) water
(d) ethyl alcohol
46. Which of the following enzymes converts starch into maltose?
(a) Maltase
(b) Diastase
(c) Zymase
(d) Invertase
47. Which of the following enzymes converts maltose into maltose?
(a) Maltase
(b) Diastase
(c) Zymase
(d) Invertase
48. Clotting of blood is possible because of
(a) WBC
(b) RBC
(c) platelets
(d) globulins
49. Which of the following is true about vitamins?
(a) Vitamins in the human body are needed in large amounts
(b) Vitamins are secreted by ductless glands
(c) Vitamins are synthesized by an rganisms
(d) Vitamins A, D, E and \(\mathrm{K}\) are fat soluble whereas vitamins of the \(B\) group and vitamin C are water soluble
50. The disease beriberi is caused by the deeficiency of vitamin
(a) \(\mathrm{A}\)
(b) \(K\)
(c) \(\mathrm{B}_1\)
(d) \(\mathrm{B}_{12}\)

28. Polymers
1. Which one of the following is a condensation polymer?
(a) Nylon
(b) Polythene
(c) PVC
(d) Teflon
2. Which one of the following is an addition polymer?
(a) Bakelite
(b) Nylon-6,6
(c) Polystyrene
(d) Terylene
3. Which of the following is a copolymer?
(a) Orlon
(b) Teflon
(c) \(\mathrm{PVC}\)
(d) PHB V
4. The monomer used to prepare superglue is
(a) vinyl chloride
(b) methyl \(\alpha\)-cyanoacrylate
(c) p-phenylene diamine
(d) diethyl carbonate
5. A polymer of butadiene and Acrylonitrile is called.
(a) Buna-S
(b) Buna- \(\mathrm{N}\)
(c) Buna-B
(d) Buna-A
6. Natural rubber is a polymer of
(a) styrene
(b) butadiene
(c) vinyl chloride
(d) isoprene
7. In which of the following pairs both are copolymers?
(a) PHBV, Bakelite
(b) Polythene, terylene
(c) Poly acrylonitrile, nylon-6,6
(d) Polystyrene, melamine
8. The polymer used in paints is
(a) Nylon
(b) Glyptal
(c) Neoprene
(d) Terylene
9. Which of the following contains biodegradable polymers only?
(a) Cellulose, dextron, PHBV
(b) Starch, PHBV, PVC
(c) Bakelite, nylon-2-nylon-6, nylon-6,6
(d) Cellulose, starch, terylene
10. Thermosetting polymer is
(a) Nylon-6
(b) Nylon-6,6
(c) Bakelite
(d) SBR
11. Nylon thread contains the polymer
(a) polyamide
(b) polyvinyl
(c) polyester
(d) polyethylene
12. Polythene, PVC, teflon and neoprene are all
(a) monomers
(b) homopolymers
(c) copolymers
(d) condensation polymers
13. Which one of the following is NOT a biodegradable polymer?
(a) Starch
(b) Cellulose
(c) Dextron
(d) Decron
14. The polymer used in making blankets (artificial wool) is
(a) polyester
(b) polyacrylonitrile
(c) polythene
(d) polystyrene
15. Trans-poly-isoprene is called
(a) Glyptal
(b) Gutta-Percha
(c) Melamine
(d) Buna-S
16. Buna-S is a
(a) monomer
(b) polymer
(c) copolymer
(d) dimer
17. Glucose is a monomer of
(a) proteins
(b) rubber
(c) plastics
(d) starch and cellulose
18. Which of the following is natural polymer?
(a) Bakelite
(b) Nylon
(c) Proteins
(d) PVC
19. Which of the following is a synthetic polymer?
(a) Starch
(b) Cellulose
(c) RNA
(d) Terelyne
20. Which of the following is an example of additional polymerisation?
(a) Proteins
(b) Teflon
(c) Nylon-66
(d) Glyptal
21. Which of the following is an example of condensation polymerisation?
(a) \(\mathrm{PVC}\)
(b) BUNA rubber
(c) Dacron
(d) Lutrex
22. Which of the following is an example of elastomers?
(a) Rubber
(b) Nylon-66
(c) PVC
(d) Bakelite
23. Which of the following is an example of fibres polymer?
(a) Rubber
(b) Nylon-66
(c) PVC
(d) Bakelite
24. Which of the following is an example of thermoplastic polymer?
(a) Rubber
(b) Nylon-66
(c) \(\mathrm{PVC}\)
(d) Bakelite
25. Which of the following is an example of thermosetting polymer?
(a) Rubber
(b) Nylon-66
(c) PVC
(d) Bakelite
26. The repeating unit of teflon is

27. The monomer of neoprene is
(a) chloroprene
(b) styrene
(c) vinyl chloride
(d) linear polymer
28. The repeating unit of polyacrylonitrile is

29. Which of the following structures represents terylene?

30. Which of the following structures represent nylon-66?

31. Glyptal resins is formed from the monomers
(a) adipic acid and hexamethylene diamine
(b) ethylene glycol and phthalic acid
(c) phenol and formaldehyde
(d) ethylene glycol and terephthalic acid
32. Nylon-6, 6 is formed from the monomers
(a) adipic acid and hexamethylenediamine
(b) ethylene glycol and phthalic acid
(c) phenol and formaldehyde
(d) ethylene glycol and terephthalic acid
33. Bakelite is formed from the monomers
(a) adipic acid and hexamethylenediamine
(b) ethylene glycol and phthalic acid
(c) phenol and formaldehyde
(d) ethylene glycol and terephthalic acid
34. Tereylene is formed from the monomers
(a) adipic acid and hexamethylenediamine
(b) ethylene glycol and phthalic acid
(c) phenol and formaldehyde
(d) ethylene glycol and terephthalic acid
35. Melamine formaldehyde resin is
(a) dimer of melamine and formaldehyde
(b) an additional polymer
(c) a copolymer
(d) a fibre type polymer
36. The monomer of nylon-6 is
(a) cyclohexane
(b) caprolactam
(c) ethylene glycol
(d) ammo acid
37. Which of the following polymers has ester linkages?
(a) Nylon
(b) Bakelite
(c) Terylene
(d) PVC
38. Which of the following polymers has amide linkages?
(a) Nylon
(b) PVC
(c) Terylene
(d) Bakelite
39. The process of vulcanization makes rubber
(a) soluble in water
(b) hard
(c) soft
(d) less elastic
40. Isoprene is a monomer of
(a) starch
(b) synthetic rubber
(c) synthetic nubber
(d) \(\mathrm{PVC}\)
41. Which one of the following polymers does not involve cross linkages?
(a) Bakelite
(b) Melamine
(c) Polythene
(d) Vucanized rubber
42. PVC stands for
(a) Polyvinyl carbinol
(b) Polyvinyl chloral
(c) Polyvinyl chloride
(d) Polyvinyl chloroform
43. Which of the following is isoprene?

44. One of the constituents in the preparation of thiokol is
(a) 1,2-dichloroethane
(b) isoprene
(c) chloroprene
(d) sulphur
45. Thiokol is a
(a) fibre
(b) plastic
(c) rubber
(d) detergent
46. Which of the following is chloroprene?
(a) \(\underset{\mathrm{Cl}}{\mathrm{CH}}=\mathrm{CH}_2-\mathrm{CH}=\mathrm{CH}_2\)
(b)
<smiles>C=CC(=C)Cl</smiles>
(c)
<smiles>CC=C(C)Cl</smiles>
(d)
<smiles>CCC(C)Cl</smiles>
47. Ziegler catalyst is
(a) chromium oxide supported over silica
(b) triethyl aluminium and titanium tetrachloride dispersed in an inert solvent
(c) alumina
(d) plantinum/paladium
48. Which of the following polymers has the empirical formula identical with that of its monomer?
(a) Teflon
(b) Nylon-66
(c) Dacron
(d) Bakelite
49. Which of the following polymers may be classified as chain growth polymers?
(a) Nylon-66
(b) Bakelite
(c) Teflon
(d) Polyester
50. Which of the following polymers may be classified as step growth polymer?
(a) Teflon
(b) PVC
(c) Polyethene
(d) Nylon-66

29. Chemistry in everyday life
1. Which of the following is NOT a detergent?
(a) Sodium lauryl sulphate
(b) \(\mathrm{n}\)-Hexyl trimethyl ammonium chloride
(c) Pentaerythrity 1 stearate
(d) Sodium butyrate
2. Which of the following is a bactericidal antibiotic?
(a) Erythromycin
(b) Ofloxacin
(c) Tetracycline
(d) Chloromphenical
3. Valium is used as
(a) tranquilizer
(b) analgesic
(c) antipyretic
(d) antibiotic
4. Codeine is
(a) tranquilizer
(b) antibiotic
(c) analgesic
(d) antacid
5. Food preservative in tomato ketchup is
(a) sodium acetate
(b) sodium benzoate
(c) sodium salicylate
(d) sodium propionate
6. The drug used to induce sleep is
(a) paracetamol
(b) bithional
(c) chloroquine
(d) equanil
7. Cetyl trimethyl ammonium chloride is an example of
(a) anionic detergent
(b) cationic detergent
(c) non-ionic detergent
(d) soap
8. Which one of the following element is not there in salvarsan?
(a) As
(b) \(\mathrm{N}\)
(c) \(\mathrm{O}\)
(d) \(\mathrm{P}\)
9. Example of antifertility drug is
(a) novestrol
(b) seldane
(c) salvarsan
(d) chloramphenicol
10. Iodex contains
(a) methyl acetate
(b) ethyl propionate
(c) methyl salicylate
(d) methyl benzoate
11. With oil or grease on cloth soap forms
(a) colloid
(b) emulsion
(c) gel
(d) \(\mathrm{sol}\)
12. Some drugs do not bind to the enzyme's active site, instead bind to a different site of enzyme. This site is called
(a) allosteric site
(b) substrate site
(c) ionic site
(d) competitive site.
13. What type of forces bind the substrate to the active site of enzyme?
(i) Ionic bonding
(ii) Hydrogen bonding
(iii)van der Waals forces
(iv) Reaction with functional group of enzymes
(a) (i), (ii) and (iv)
(b) (i). (iii) and (iv)
(c) (i), (ii) and (iii)
(d) (i), (ii), (iii) and (iv)
14. Drugs that bind to the receptor site and inhibit its natural function are called
(a) agonistic drugs
(b) antagonistic drugs
(c) antimicrobial drugs
(d) allosteric drugs.
15. A drug which acts as antipyretic as well as analgesic is
(a) chloroquin
(b) penicillin
(c) chlordiazepoxide
(d) 4-acetamidophenol.
16. Which of the following can be used as an analgesic without causing addiction?
(a) Morphine
(b) Aspirin
(c) Heroin
(d) Codeine
17. Which of the following will not act as a tranquilizer?
(a) Equanil
(b) Analgin
(c) Meprobamate
(d) Chlordiazepoxide
18. Antihistamines are not helpful
(a) in curing nasal allergies
(b) in treating rashes caused by itching
(c) in bringing down acute fever
(d) in vasodilation.
19. The main cause of acidity in the stomach is
(a) release of extra gastric acids which decrease the \(\mathrm{pH}\) level
(b) indigestion and pain in large intestine
(c) increase the \(\mathrm{pH}\) level in the stomach
(d) release of extra bile juice which increases alkaline medium in stomach.
20. The chemical substances used to bring down body temperature in high fever are known as
(a) analgesics
(b) antipyretics
(c) antihistamines
(d) tranquilizers.
21. Match-the drugs in column I with the examples given in column II and mark the appropriate choice.
Column I Column II
(A) Antibiotic \(\quad\) (i) Codeine
(B) Antiseptic (ii) Phenelzine
(C) Analgesic \(\quad\) (iii) Chloramphenicol
(D) Tranquilizer
(iv) Chloroxylenol
(a) (A) \(\Rightarrow\) (i), (B) \(\rightarrow\) (iii), (C) \(\rightarrow\) (ii), (D) \(\rightarrow\) (iv)
(b) (A) \(\rightarrow\) (iv), (B) \(\rightarrow\) (ii), (C) \(\rightarrow\) (iii), (D) \(\Rightarrow\) (i)
(c) (A) \(\rightarrow\) (ii), (B) \(\rightarrow\) (iv), (C) \(\rightarrow\) (i), (D) \(\rightarrow\) (iii)
(d) (A) \(\rightarrow\) (iii), (B) \(\rightarrow\) (iv), (C) \(\rightarrow\) (i), (D) \(\rightarrow\) (ii)
22. Barbiturates acts as
(a) hypnotic i.e., sleep producing agents
(b) non-narcotic analgesics
(c) activator of neurotransmitters
(d) antiallergic drugs
23. Antiseptics are the chemicals which either or the growth of microorganisms and are applied to the
(a) kill, prevent, living tissues
(b) kill, prevent, non-living objects
(c) increase, decrease, living tissues
(d) kill, increase, non-living tissues
24. The term 'broad spectrum antibiotics' means
(a) bactericidal antibiotics
(b) bacteriostatic antibiotics
(c) which kill or inhibit a wide range of gram-ve and gram +ve bacteria
(d) which kill or inhibit all types of gram +ve bacteria.
25. The main constituents of dettol are
(a) chloramphenicol + glycerol
(b) 2-3\% solution of iodine in alcohol
(c) \(0.2 \%\) solution of phenol
(d) chloroxylenol and terpineol
26. The antibiotic which is effective against certain strains of cancer cells,
(a) dysidazirine
(b) sulphanilamide
(c) vancomycin
(d) ofloxacin.
27. Which of the following is not an antidepressants?
(a) Iproniazid
(b) Phenelzine
(c) Equanil
(d) Salvarsan
28. What is tincture of iodine?
(a) \(2-3 \%\) solution of iodine in alcohol-water mixture.
(b) A mixture of iodine in chloroxylenol.
(c) A mixture of \(0.2 \%\) phenol and \(2-3 \%\) iodine in water.
(d) 2-3\% solution of iodine in potassium iodide
29. Name an artificial sweetener which is derivative of sucrose.
(a) Saccharine
(b) Sucrolose
(c) Sucrobenzamide
(d) Aspartame
30. The main difference between bathing and washing soap is
(a) bathing soaps are potassium salts of fatty acids while washing soaps are sodium salts of fatty acids
(b) bathing soaps are sodium salts of fatty acids while washing soaps are potassium salts of fatty acids
(c) bathing soaps are cationic in nature while washing soaps are anionic
(d) bathing soaps are calcium salts of fatty acids while washing soaps are magnesium salts of fatty acids.
31. Which of the following is not used as a food preservative?
(a) Sodium salt of benzoic acid
(b) Sodium salt of sorbic acid
(c) Sodium salt of propanoic acid
(d) Sodium salt of palmitic acid
32. Which of the following is not a food additive?
(a) Preservatives
(b) Sweetening agents
(c) Flavours
(d) Oxidants
33. Which is not true for a detergent molecule?
(a) It has a non-polar organic part and a polar group.
(b) It is not easily biodegraded.
(c) It is a sodium salt of fatty acid.
(d) It is a surface active reagent.
34. Which of the following is not a true statement about the detergents?
(a) Anionic detergents are sodium salts of sulphonated long chain alcohols or hydrocarbons.
(b) Cationic detergents are quarternary ammonium salts of amines with acetates, chlorides or bromides as an ions.
(c) Non-ionic detergents do not contain any ion in their constitution.
(d) Detergents containing branched hydrocarbon chains are biodegradable.
35. Identify the hydrophilic and hydrophobic parts in the following non-ionic detergent present in liquid detergents and wetting agents.

