BOOK: THERMAL EXPANSION, CALORIMETRY & HEAT TRANSFER
- Calorimetry
- Thermal Expansion
- Conduction
- Convection
- Radiation
1. CALORIMETRY
CALORIMETRY
Calorimetry is that branch of heat which deals with its measurement of heat.
SPECIFIC HEAT
This is also called ' Heat Inertia' of a substance
Specific Heat of solid & Liquid (s) : If a solid or a liquid is heated till the change of the state, there is no change in their volume hence the work done is zero. Hence there is only one specific heat of solids and liquids which is constant.
Definition :
1. The amount of heat needed for an unit increase in the temperature of unit mass of a solid or liquid is called it's specific heat
Unit : kilocalorie \(/ \mathrm{kg}-{ }^{\circ} \mathrm{C}\) or calorie \(/ \mathrm{g}-{ }^{\circ} \mathrm{C}\)
\(1 \mathrm{Kcal} / \mathrm{Kg}-{ }^{\circ} \mathrm{C}=1 \mathrm{Cal} / \mathrm{g}^{\circ} \mathrm{C}\)
2. If mass of the body is ' \(m\) ' and specific heat is ' \(s\) ' then amount of heat needed to increases it's temperature by \(\mathrm{dT}\) is given by \(\mathrm{dQ}=\operatorname{msdT}\)
3. Specific heat of water \(=1 \mathrm{kcal} / \mathrm{kg}-{ }^{\circ} \mathrm{C}=1 \mathrm{Cal} / \mathrm{g}-{ }^{\circ} \mathrm{C}=4.18 \times 10^{3} \mathrm{~J} / \mathrm{kg}-{ }^{\circ} \mathrm{C}\)
4. Specipic heat of ice \(=\) specific heat of steam \(=0.5 \mathrm{~K} \mathrm{Cal} / \mathrm{K}_{3}{ }^{\circ} \mathrm{C}=0.5 \mathrm{Cal} / \mathrm{g}^{\circ} \mathrm{C}\)
5. Kelvin can also be used instead of \({ }^{\circ} \mathrm{C}\) is size of both uints is same.
THERMAL CAPACITY
(1) Amount of heat needed to increase the temperature of a substance (any amount) by \(1^{\circ} \mathrm{C}\) is called thermal capacity of that substance.
(2) Thermal capacity \(=(\) mass of body \() \times(\) specific heat \() \Rightarrow \mathrm{H}_{\mathrm{c}}=\mathrm{ms}\)
(3) Unit \(=\) calorie \(/{ }^{\circ} \mathrm{C} \quad\) or \(\quad \mathrm{Kcal} /{ }^{\circ} \mathrm{C}\)
(4) Thermal capacity is given by reciprocal of slope of heat temperature curve.as
\(\mathrm{H}_{\mathrm{c}}=\mathrm{mS}=\frac{\mathrm{dQ}}{\mathrm{dT}} \)
Heat capacity at point ' \(\mathrm{p}^{\prime}=\frac{1}{\tan \theta}=\cot \theta\)
(5) Heat capacity in an isothermal process is infinite \((\infty)\). e.g. process of melting and vaporisation
(6) If heat capacity of a body is \(\mathrm{H}_{\mathrm{c}}\), then heat needed to rise it's temperature by \(\mathrm{d} \theta\) is, \(\mathrm{dQ}=\mathrm{H}_{\mathrm{c}} \mathrm{d} \theta\)
WATER EQUIVALENT OFA BODY
(1) If \(\mathrm{m}\) gram of a substance is given \(Q\) amount of heat which rises its temperature by \(\Delta T\). Now if on giving same amount of heat temperature of \(w\) gram of water is also increased by \(\Delta \mathrm{T}\) then \(w\) is called water equivalent of body of mass \(m\).
(2) The value of water equivalent of a body is same as it's heat capacity. The difference is only in units. e.g If heat capacity of a body is \(\mathrm{m}\) calorie \(/{ }^{0} \mathrm{C}\) then it's water equivalent will be \(\mathrm{m}\) gram.
Physical meaning: The same amount of heat has to be given to a body for increasing it's temperature by \(\mathrm{dT}\) as needed for quantity of water equal to it's water equivalent by same temperature range.
Phase transformation curve
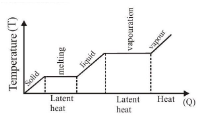
LATENT HEAT
Latent heat of fusion of a substance is the quantity of heat (in kilocalories) required to change its 1 \(\mathrm{kg}\) mass from solid to liquid state at its melting point For ice latent heat of fusion \(=80 \mathrm{kilocal} / \mathrm{kg}=80 \mathrm{cal} / \mathrm{gm}\).
Latent heat of vaporization of a substance is the quantity of heat required to change its \(1 \mathrm{~kg}\) mass from liquid to vapour state at its boiling point.
For water latent heat of vaporisation \(=540 \mathrm{kilocal} / \mathrm{kg}=540 \mathrm{cal} / \mathrm{gm}\).
