CRASH COURSE MHT-CET CHEMISTRY
1. CONCEPT MOLE CONCEPT
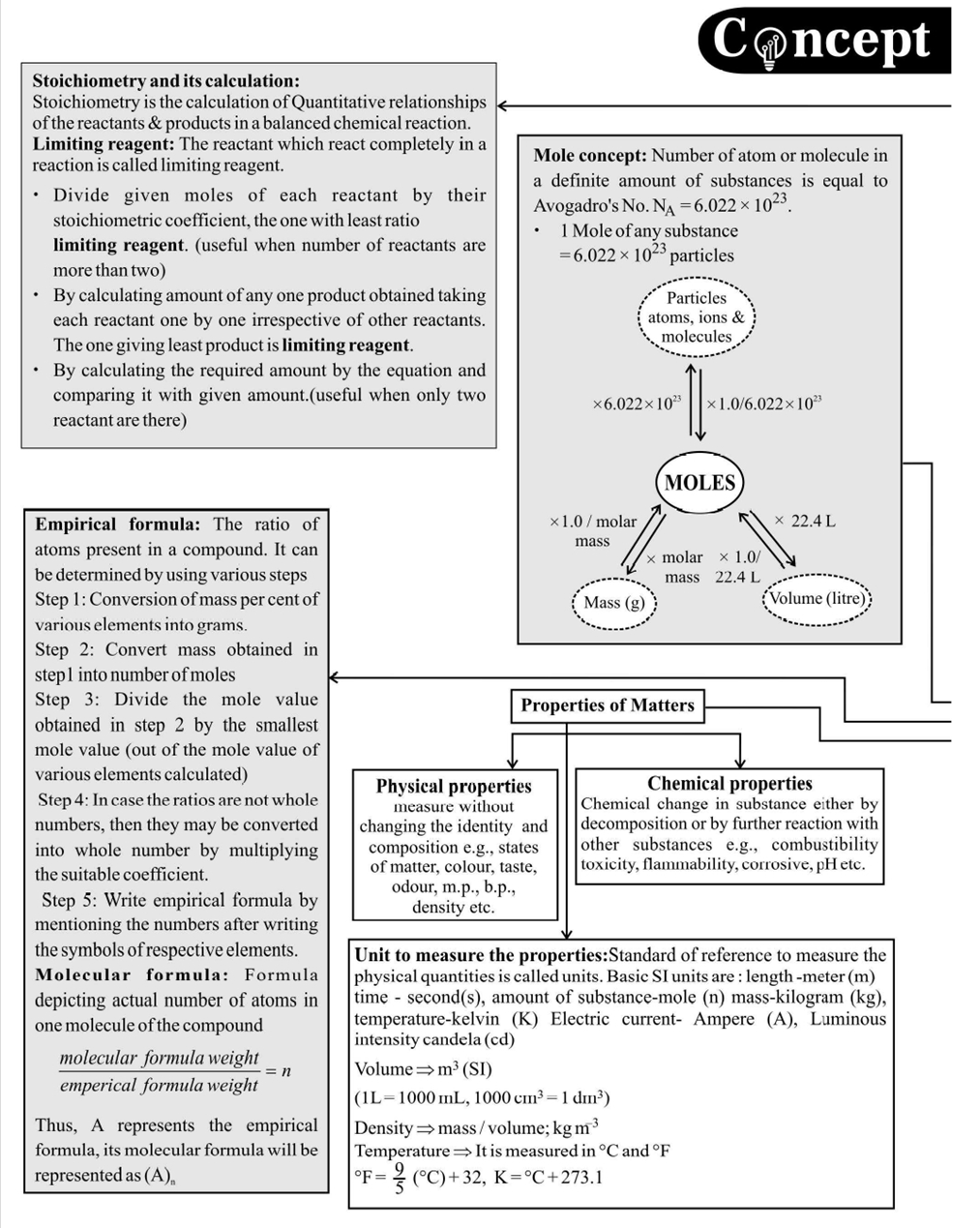
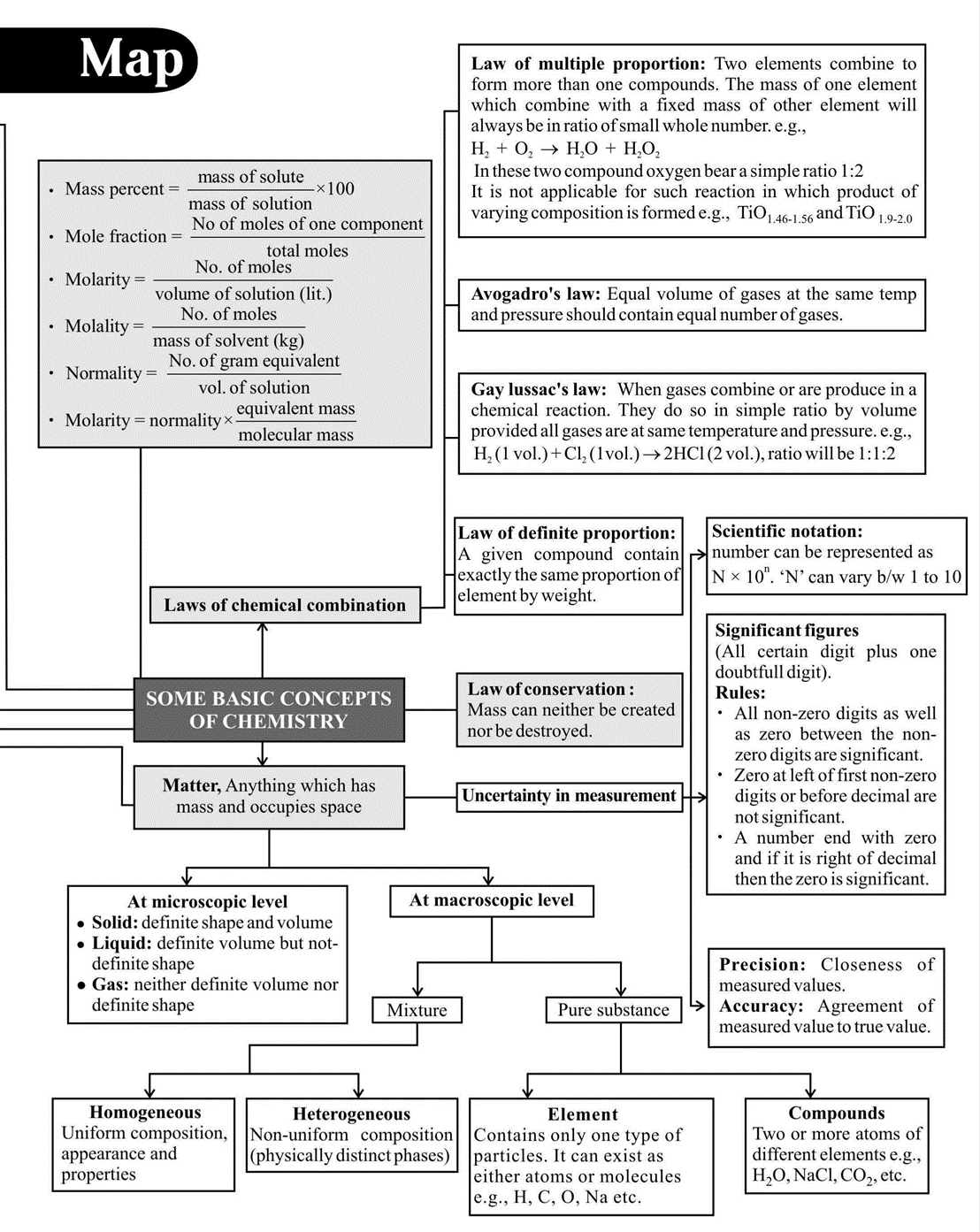
Important Points to Remember
- Dulong and Pettit's method
Atomic mass \(\times\) Specific heat \(=6.4\) (approx.)
This law is applicable to solid elements only except Be, B, C and \(\mathrm{Si}\)
- On diluting a solution, eq, meq, mole or \(\mathrm{m}\) mole of solute do not change however \(\mathrm{N}\) and \(\mathrm{M}\) change (decrease).
- Molality, \% by weight, mole fraction do not depend on temperature since they involve weights.
- Normality, molarity, percent of volume; percent by strength, and strength are temperature dependent. They decrease with increase in temperature since the volume of solution increases with \(\mathrm{T}\).
- Sometimes, the term formality is used in place of molarity.
- Standard solution is one whose \(\mathrm{N}\) or \(\mathrm{M}\) is known.
- Normality: It is defined as number of equivalents of a solute present in one litre of solution. \(\mathrm{N}\) = Equivalent of solute/ Volume of solution (L)
\(\mathrm{N}=\frac{\text { Weight of solution }}{\text { Equivalent weight of solute } \times \mathrm{V}_{\text {sol }}(\mathrm{inL})}\)
\(\mathrm{N}=\frac{\mathrm{W}}{\mathrm{E} \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{L})}=\frac{\mathrm{W} \times 1000}{\mathrm{E} \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{mL})}\)
- Molarity: It is defined as the moles of solute present in one litre of solution.
\(\mathrm{M}=\frac{\text { Moles of solute }}{\text { Volume of solution in litre }}\)
\(M=\frac{\text { Weight of solute }}{\text { Molecular weight of solute } \times \mathrm{V}_{\text {sol }}(\mathrm{in} \mathrm{L})}\)
- Molality: Moles of solute present in \(1 \mathrm{~kg}\) of solvent.
Molality \(=\) Moles of solute/ Weight of solvent in \(\mathrm{kg}\)
D Vapour density method
Tips to Problem Solving
\(n\)-Factor
Molecular weight \(=2 \times\) vapour density
Equivalent weight \(=\) atomic weight \(/ n\)-factor
In case of acid/base the \(n\)-factor is basicity/acidity (i.e. number of dissociable \(\mathrm{H}^{+}\)ions/number of dissociable \(\mathrm{OH}^{-}\)ions) and in case of oxidizing agent/reducing agent, \(n\) factor is number of moles of electrons gained/lost per mole of oxidizing agent/reducing agent.
" Molality \((m)\) expressed in terms of mole fraction of solute \(\left(X_{1}\right)\) is given by relation: \(m=\frac{1000 X_{1}}{\left(1-X_{1}\right) m_{2}}\) where \(\mathrm{m}_{2}\) is the molar mass of the solvent.
" Molality \((m)\) is related to molarity \((M)\) by relation : \(\mathrm{m}=\frac{1000 \mathrm{M}}{1000 \mathrm{~d}-\mathrm{Mm}_{1}}\) where \(d\) is the density \(\left(\mathrm{g} \mathrm{mL}^{-1}\right)\) and \(m 1\) is the molar mass of the solute
" Percentage:
\%by weight \(=\frac{\text { Weight of solute }}{\text { Weight of solute }} \times 100\)
\%by volume \(=\frac{\text { volume of solute }}{\text { Volume of solution }} \times 100\)
\(\%\) by \((\) W \(/ \mathrm{V})=\frac{\text { Weight of solute }}{\text { Volume of solution }} \times 100\)



